Navigating the Dual Threat: Cryptococcal and Tubercular Co-infections in PLHIV
JASPI September 2024/ Volume 2/Issue 3
Copyright: © Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Kumar B, Kumar D, Meena DS, Kanagiri T, et al.Navigating the Dual Threat: Cryptococcal and Tubercular Co-infections in PLHIV. JASPI. 2024;2(3):-40-45 DOI: 10.62541/jaspi039
ABSTRACT
Co-infections of Cryptococcosis and tuberculosis (TB) represent a significant clinical challenge, particularly in people living with HIV, due to their compounded impact on an already compromised immune system. Cryptococcus neoformans and Mycobacterium tuberculosis exploit immune dysfunction, often resulting in severe, disseminated infections with overlapping clinical symptoms, which complicates diagnosis and delays appropriate treatment. A 55-year-old HIV-positive male, recently initiated on ART (TLD regimen) was admitted with fever, headache, vomiting, irritability, and altered behavior. Poor adherence to ART and a CD4 count of 210 cells/cumm raised concerns about a CNS opportunistic infection, possibly cryptococcal meningitis and immune reconstitution inflammatory syndrome (IRIS). Examination revealed fever, irritability, neck stiffness, and positive Kernig’s signs. Cryptococcal antigen was positive, and imaging suggested both cryptococcal and tubercular meningitis. Bronchoalveolar lavage confirmed tuberculosis. The patient was treated with liposomal amphotericin B, fluconazole, and rifampicin-sparing antitubercular therapy. After showing clinical improvement, he was discharged with dual therapy and stabilized after 3 weeks of hospitalization. Our findings emphasize the importance of comprehensive clinical guidelines and multidisciplinary management to address the complexities of treating this co-infection. Our case review underscores the need for integrated diagnostic and therapeutic protocols, timing of initiation of ART, and comprehensive management strategies to improve outcomes for this vulnerable patient population.
KEYWORDS: Antiretroviral therapy, cryptococcal meningitis, disseminated tuberculosis, IRIS, opportunistic infections, PLHIV
INTRODUCTION
Human Immunodeficiency Virus (HIV) significantly weakens the immune system, rendering individuals vulnerable to a range of life-threatening opportunistic infections (OI), particularly in the advanced stage when CD4+ T-cell counts fall below 200 cells/cumm. Among these, tuberculosis (TB) and cryptococcal infection stand out as significant causes of morbidity and mortality.
Tuberculosis caused by Mycobacterium tuberculosis is the second most common infectious cause of mortality after COVID-19.1 The synergistic interaction between HIV and TB exacerbates the clinical course of both diseases. HIV patients are more likely to present with disseminated and extrapulmonary forms of TB, which complicates both diagnosis and treatment due to atypical presentations and increased drug resistance. The WHO estimated that there were almost 10.6 million new cases of TB in 2022. Approximately 1.3 million people died from TB in 2022; among them, 167000 were people living with HIV (PLHIV).1
Cryptococcal infection, primarily caused by Cryptococcus neoformans, is a fatal fungal disease that commonly affects the central nervous system (CNS), leading to cryptococcal meningitis (CM), but can also involve other organs. This infection is particularly deadly in patients with advanced HIV and low CD4+ counts. Despite advancements in antiretroviral therapy (ART), cryptococcal meningitis continues to be a significant cause of death in HIV-infected populations, especially in resource-limited settings. Mortality from CM is highest in developing low-income countries, accounting for around 70%–80% in low-income versus only 20%–30% in high-income countries.2
Our case report highlights the concurrent presentation of tuberculosis and cryptococcal infection in an HIV-infected patient, illustrating the diagnostic and therapeutic challenges and the clinical management strategies for handling such co-infections in the context of HIV.
CASE PRESENTATION
A 55-year-old male was found to be seropositive for HIV when he was evaluated at a remote hospital two months ago for complaints of fever, weight loss, and loss of appetite. He was immediately initiated on ART (TLD regimen) without OI workup. Now, he presented to us with complaints of fever, headache and vomiting for the last 15 days, with irritability and altered behaviour over the previous two days. There is no history of seizure, visual blurring or spots, focal neurological deficit, cough, shortness of breath or hemoptysis, abdominal pain or loose motion. Further history revealed his poor adherence to the ART regimen. His CD4 count was 210 cells/cumm, and plasma viral load status was unknown. On examination, he was conscious but not cooperative, irritable, and febrile (Temp100.20F) with a normal blood pressure of 126/76mmHg, respiratory rate of 18/min, and pulse rate of 89/min. Some sub centimetric-size lymph nodes were palpable in cervical level V and the inguinal region. Neck rigidity and Kernig’s signs were positive, while the rest of the systemic examination was normal. A diagnosis of a CNS opportunistic infection with a possible Immune Reconstitution Inflammatory Syndrome (IRIS) was suspected, so antiretroviral therapy (ART) was stopped immediately. The patient was promptly referred for brain imaging and an opportunistic infection workup.
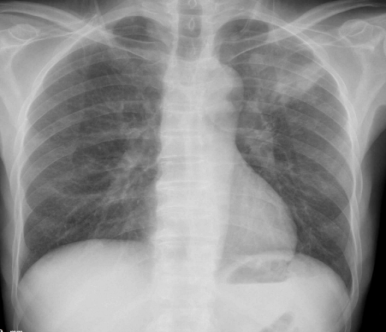
On day 1, all basic investigations, including hemogram, kidney function test, liver function test, urine routine microscopy, serum electrolytes, and blood glucose, were within normal limits, except for a slight A: G reversal (3.3:4.1). Inflammatory markers were elevated, with an ESR of 49 and a hs-CRP level of 11.56. A chest X-ray revealed opacity in the left upper lobe [Figure 1A]. The opportunistic infection workup was notably positive for cryptococcal antigen (1:320 titer by IMMY CrAg® LFA), while tests for Hepatitis B, Hepatitis C, toxoplasma, VDRL, and CMV were negative. Both blood and urine cultures were sterile.
To confirm the etiology, we planned for a CSF analysis; however, despite multiple attempts, a lumbar puncture could not be performed. Considering the high mortality associated with cryptococcal meningitis, therapy was initiated immediately with intravenous liposomal amphotericin B (3 mg/kg body weight) and high-dose oral fluconazole (1200 mg/day), following the recent WHO guidelines for resource-limited settings, along with all necessary precautionary measures.
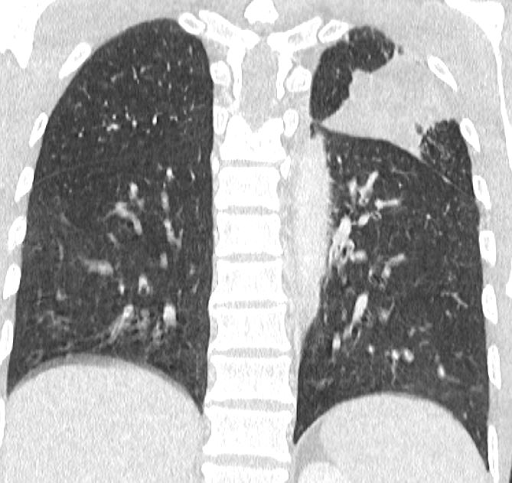
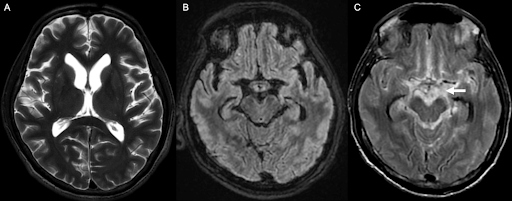
With the high suspicion of tuberculosis, a contrast-enhanced CT (CECT) of the thorax and abdomen was ordered to identify the source of infection. The scan revealed focal consolidation in the left upper and lower lobes, multiple centrilobular nodules with a ‘tree-in-bud’ pattern, and necrotic mediastinal and abdominal lymphadenopathy, consistent with active disseminated tuberculosis [Figure 1B-D]. The contrast-enhanced MRI brain report showed linear leptomeningeal enhancement in both cerebral and cerebellar hemispheres with extensive basal exudates with a possibility of either tubercular or cryptococcal infection [Figure 2A-C]. Additionally, linear enhancement with mild nodularity was observed along the entire length of the spinal cord. These features were suggestive of active tubercular meningitis.
Figure 1: Radiological images of the chest and abdomen. (A) showing chest Xray PA view having left upper zone heterogeneous opacity; (B-D) showing CECT of chest and abdomen having focal area of consolidation in left upper and lower lobes and multiple centrilobular nodules with tree in bud configuration with necrotic mediastinal and abdominal lymphadenopathy, in favor of active disseminated tuberculosis.
[A] [B]
[C] [D]
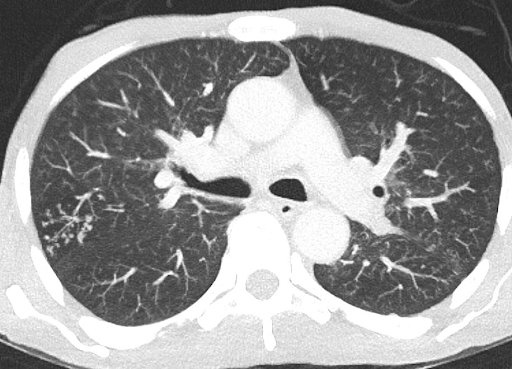
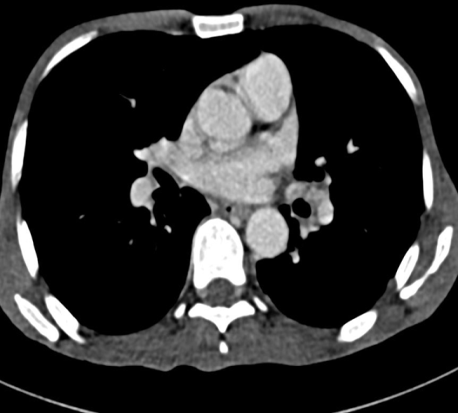
Figure 2: MR images of the brain. (A) Axial T2 weighted image shows mild lateral ventricular prominence; (B) Axial T2-FLAIR (fluid-attenuated inversion recovery); (C) Post-contrast T2-FLAIR sequence depicts significant contrast enhancement in basal cisterns (arrow), suggestive of tubercular/cryptococcal meningitis.
Since the patient could not produce sputum, bronchoscopy and bronchoalveolar lavage (BAL) were performed. The BAL cytology revealed 83% neutrophils and 13% macrophages. The analysis was positive for CBNAAT (with no rifampicin resistance detected) and galactomannan, indicating both tubercular and fungal etiologies. Other BAL analyses, including Gram stain, aerobic bacterial culture, KOH preparation, and fungal culture, did not reveal any additional findings. Rifampicin-sparing antitubercular therapy was initiated, which included isoniazid,
ethambutol, pyrazinamide, and levofloxacin, due to the interaction between rifampicin and fluconazole. The patient showed improvement with the dual therapy.
After a 2-week induction phase with intravenous liposomal amphotericin B, the patient continued consolidation therapy with 800 mg of oral fluconazole. The modified antitubercular treatment was maintained, and the patient was discharged in a stable condition with a fully oriented mental status after 3 weeks of hospitalization.
DISCUSSION
According to WHO estimates of global HIV epidemics for 2023, around 39 million (33.1–45.7 million) people were living with HIV, and approximately 1.3 million (1.0–1.7 million) people acquired new HIV infections in 2022. Of these, HIV-related causes accounted for 48.46% of deaths, totaling 630,000 (480,000–880,000) deaths, despite the availability of effective antiretroviral therapy.6 India has the third highest burden of people living with HIV (PLHIV) in the world. Estimates for 2021 indicate a prevalence of 0.21%, with 2,401,284 (1,992,058–2,906,772) people infected with HIV and 62,967 (36,715–104,058) new infections acquired that year. Among these, 66.65% (41,968; 26,499–67,451) of the deaths were attributable to HIV-related causes.2,7
Human immunodeficiency virus (HIV) has a very high propensity to infect neural tissues. It has both neuroinvasive and neurovirulent properties. Clinically evident neurological dysfunction manifests in around 60% of PLHIV, and in 10-20% of them, neurological symptoms appear as a first manifestation of HIV.8-9 Neurological manifestations of HIV occur due to direct viral invasion in brain cells, toxicity of antiretroviral drugs and opportunistic infections.10-11 Life-threatening opportunistic infections (OIs) play a crucial role in HIV-related morbidity and mortality. OIs are the consequences of declining immunity (CD4 count < 200 cells/cumm) in PLHIV and depend upon the endemicity of the causative organism.2
Tuberculosis and cryptococcosis are serious infections affecting both immunocompromised and immunocompetent individuals. These infections can involve any organ in the body. HIV/AIDS is a common form of immunodeficiency, and these infections are often associated with HIV/AIDS. Impaired immunity from factors such as corticosteroid use, immunosuppressive and chemotherapeutic drugs, malnutrition, substance abuse, diabetes mellitus, solid organ or stem cell transplantation, malignancy, and autoimmune diseases can also lead to opportunistic infections, either alone or as co-infections. While tuberculosis and cryptococcal co-infection are not uncommon, it is often missed. These infections share certain clinical features, including a predilection for CNS involvement, endemicity, chronicity (subacute to chronic forms), imaging characteristics, and initial CSF findings.12
Retrospective analysis of TB-cryptococcal co-infection in a Chinese database of >50 years (1965-2016) revealed 197 cases of co-infection only, with more than half of the cases 56.3% were reported after 2010, and among them, CNS involvement in the form of meningitis found in 54% of cases.13 Another retrospective analysis was carried out for this co-infection in Taiwan between 1993-2006. They found 23 patients with cryptococcosis and TB co-infection, out of which 48% (11 patients) were seropositive for HIV.14 One case series between 2007 and 2019 at a tertiary care centre in southern India reported 5 cases of culture-proven TB-cryptococcal co-infection. Four patients were HIV infected, and one was on immunosuppressive therapy with azathioprine and steroid for sclerosing glomerulonephritis.15 One study found that HIV patients with both TB and cryptococcal meningitis had a mortality rate of approximately 55%, compared to 30-40% for those with cryptococcal meningitis alone.16
Many diagnostic and therapeutic challenges exist with tuberculosis and cryptococcal co-infection, which contributes to an underestimation of its actual prevalence. Standard assays for tuberculosis diagnosis, such as microscopy and culture, have poor sensitivity and specificity in HIV-infected individuals and take longer to detect the Mycobacterium tuberculosis complex. The development of CBNAAT and CBNAAT Ultra has significantly improved our ability to diagnose TB more quickly and with better sensitivity and specificity. The lateral flow lipoarabinomannan assay for urine, recently recommended by WHO for diagnosing TB in PLHIV, is a promising point-of-care test, though availability remains a significant concern. In contrast, we have the highly sensitive and specific Cryptococcal Antigen Lateral Flow Assay (CRAg-LFA) for cryptococcal disease, which serves as an excellent point-of-care diagnostic tool.
Another significant challenge is the therapeutic approach. The first issue is the use of corticosteroids as an adjuvant therapy. While corticosteroids can help reduce morbidity in tubercular meningitis, they can worsen outcomes in cryptococcal meningitis. The second challenge involves drug interactions between rifampicin and antifungal agents. Despite knowing that rifampicin, with its excellent CNS penetration, is a crucial drug in treating tubercular meningitis (TBM), we had to opt for a rifampicin-sparing antitubercular regimen to avoid drug interactions and potential therapy failure. The patient was started on ART without further workup for opportunistic infections. In this case, the possibility of IRIS cannot be ruled out, as the symptoms appeared after the initiation of ART. IRIS is a hyperinflammatory reaction against latent infections following ART initiation. The improvement in CD4 T cells and subsequent immune recovery triggers this reaction.17
Both tuberculosis and cryptococcosis are WHO stage 3 and 4 AIDS-defining illnesses, and they have a severe impact on morbidity and mortality in both HIV-positive and HIV-negative individuals. It is crucial to thoroughly evaluate patients in conditions where these infections may occur and address the diagnostic and therapeutic challenges. Early diagnosis and appropriate treatment are essential to improving patient outcomes.
Our patient responded well to the tailored treatment regimen, with gradual clinical improvement observed. Regular follow-up and monitoring were essential to manage potential complications and ensure adherence to the complex treatment regimen. A multidisciplinary approach that includes careful diagnostic evaluation, tailored therapeutic regimens, and vigilant monitoring for complications is essential for improving outcomes.
CONCLUSIONS
This case report describes the significant challenges in diagnosing and managing HIV patients co-infected with TB and cryptococcal meningitis. It highlights the need for vigilant diagnostic evaluation, careful consideration of drug interactions and toxicities, and strategic timing of ART initiation to manage IRIS effectively. The successful outcome reinforces the importance of a comprehensive, patient-centered approach in managing such complex co-infections.
INFORMED CONSENT
Written informed consent was obtained from the patient. Confidentiality of the patient was maintained in the article.
CONFLICTS OF INTEREST STATEMENT
The authors declare no conflict of interest.
SOURCE OF FUNDING
None
AUTHOR’S CONTRIBUTION
BK: Conceptualization; Writing the draft
DK: Conceptualization; Writing the draft; Investigation; Methodology; Validation; Review & Editing
DSM: Investigation Methodology; Validation; Review & Editing
TK: Conceptualization; Writing the draft
GSB: Investigation; Methodology; Supervision
NKM: Investigation; Methodology; Validation; Review & Editing
REFERENCES
WHO. Global Tuberculosis Report 2023. Geneva: World Health Organization.
Accessed September 19, 2024. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023
NACO. National Guidelines for HIV Care and Treatment 2021. New Delhi: National AIDS Control Organization, Ministry of Health and Family Welfare, Government of India.
Accessed September 19, 2024. https://naco.gov.in/sites/default/files/National_Guidelines_for_HIV_Care_and_Treatment_2021.pdf
Voelz K, May RC. Cryptococcal interactions with the host immune system. Eukaryot Cell. 2010;9(6):835-46.
Chai Q, Wang L, Liu CH, Ge B. New insights into the evasion of host innate immunity by Mycobacterium tuberculosis. Cell Mol Immunol. 2020;17(9):901-13.
Rutakingirwa MK, Cresswell FV, Kwizera R, et al. Tuberculosis in HIV-Associated Cryptococcal Meningitis is Associated with an Increased Risk of Death. J Clin Med. 2020;9(3):781.
van Schalkwyk C, Mahy M, Johnson LF, Imai-Eaton JW. Updated Data and Methods for the 2023 UNAIDS HIV Estimates. J Acquir Immune Defic Syndr. 2024;95(1S):e1-e4.
NACO. India HIV Estimates 2021: Fact Sheet. New Delhi: National AIDS Control Organization, Ministry of Health and Family Welfare, Government of India.
Accessed September 19, 2024. https://naco.gov.in/sites/default/files/India%20HIV%20Estimates%202021%20_Fact%20Sheets__Final_Shared_24_08_2022_0.pdf
Martinez-Navio JM. Neurological complications during HIV infection. Explor Neuroprot Ther. 2021;1:19-32.
Sonkar SK, Gupta A, Atam V, Chaudhary SC, Tripathi AK, Sonkar GK. Clinical Profile of Neurological Manifestation in Human Immunodeficiency Virus-positive Patients. N Am J Med Sci. 2012;4(11):596-9.
Valcour V, Sithinamsuwan P, Letendre S, Ances B. Pathogenesis of HIV in the central nervous system. Curr HIV/AIDS Rep. 2011;8(1):54-61.
Modi G, Mochan A, Modi M. Neurological Manifestations of HIV. In: Okware S, eds. Advances in HIV and AIDS Control. London: IntechOpen; 2018. https://www.intechopen.com/chapters/63934
Jolobe OMP. An opportunity also for comparing rates of tuberculosis/cryptococcosis co-infection. QJM. 2022;115(5):335.
Fang W, Zhang L, Liu J, et al. Tuberculosis/cryptococcosis co-infection in China between 1965 and 2016. Emerg Microbes Infect. 2017;6(8):e73.
Huang CT, Tsai YJ, Fan JY, Ku SC, Yu CJ. Cryptococcosis and tuberculosis co-infection at a university hospital in Taiwan, 1993-2006. Infection. 2010;38(5):373-379.
Suresh CS, Ninan MM, Zachariah A, Michael JS. Cryptococcosis with Tuberculosis: Overlooked Co-infections. J Glob Infect Dis. 2021;13(3):139-41.
Jarvis JN, Meintjes G, Williams A, Brown Y, Crede T, Harrison TS. Adult meningitis in a setting of high HIV and TB prevalence: findings from 4961 suspected cases. BMC Infect Dis. 2010;10:67.
Thapa S, Shrestha U. Immune Reconstitution Inflammatory Syndrome. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. https://www.ncbi.nlm.nih.gov/books/NBK567803/.
Submit a Manuscript:
Copyright © Author(s) 2024. JASPI- Journal of Antimicrobial Stewardship Practices and Infectious Diseases.

