Anti-fungal Consumption by Days of Therapy (DOT) Metric in a Teaching Hospital: A Step towards Anti-fungal Stewardship
JASPI December 2024/ Volume 2/Issue 4
Copyright: © Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Patel R, Chauhan B, Modi C, Patel N.Anti-fungal Consumption by Days of Therapy (DOT) Metric in a Teaching Hospital: A Step towards Anti-fungal Stewardship. JASPI. 2024;2(4):17-24 DOI: 10.62541/jaspi050
ABSTRACT
Background: Anti-fungal stewardship (AFS) remains an under-explored aspect within antimicrobial stewardship programs, particularly in developing nations. Understanding the trend of anti-fungal agent consumption at our institute will assist with implementing an anti-fungal stewardship program (AFSP) soon. This study was carried out to provide benchmarking data on anti-fungal consumption, compare the consumption of anti-fungal agents among various hospital locations, and identify target locations for AFS.
Methods: After the approval of the Institutional Ethics Committee (IEC), a retrospective observational study was conducted at Shree Krishna Hospital, Karamsad. Antifungal consumption was calculated using the Days of Therapy (DOT) metric in adult inpatients in whom systemic antifungal agents (amphotericin B, anidulafungin, caspofungin, fluconazole, flucytosine, and voriconazole) were administered from 2020 to 2022. Data of DOT were captured through the Hospital Information System (HIS) and analysed using Microsoft Excel for Mac version 16.75 (23070901).
Results: Over three years, one patient was prescribed anti-fungal agents per 1000 admissions. Overall anti-fungal consumption in the last three years was 2.96 DOT/1000 Patient Days (PD). The consumption has increased over three years from 2.63 DOT/1000 PD in 2020 to 3.81 DOT/1000 PD in 2022. Fluconazole (2.48 DOT/1000 PD) was the most common anti-fungal agent consumed. The highest anti-fungal use was observed in critical care units (7.48 DOT/1000 PD).
Conclusion: Our study showed increased consumption of antifungal agents over three years. A prospective study is recommended to assess the rationality of antifungal agent prescriptions in locations with high consumption.
KEYWORDS: Anti-fungal; Anti-fungal stewardship; AFSP; Days of therapy; DOT
INTRODUCTION
Fungal infections pose a significant health burden globally. In different geographical locations, the prevalence of fungal diseases differs based on climate, environmental conditions, socioeconomic factors, and patient-related factors.1 Fungal infections affect over a billion individuals worldwide and are associated with significant mortality rates. The deaths attributed to fungal diseases are comparable to those caused by
tuberculosis and exceed those from malaria by more than threefold.2 The current population of India is 1,457,001,793 as of Friday, December 20, 2024. This makes India the most populated country in the world, representing 17.78 % of the world population.3 Around 4.4% of India’s population suffers from serious fungal diseases.4 India, a tropical country, features diverse geographical landscapes such as mountains, plains, plateaus, and numerous rivers. Extensive oceanic boundaries on three sides surround it.1 All these factors make fungal infections endemic in India, significantly affecting public health.5 Anti-fungal medications are essential for treating a broad spectrum of fungal infections, ranging from skin-level (superficial) to deeper tissue infections (subcutaneous) and potentially life-threatening systemic fungal infections throughout the body.6 However, due to excessive and improper use of anti-fungal medications, certain fungi have developed resistance to these drugs, making it difficult to treat fungal infections effectively, often requiring alternative treatment strategies or higher doses to combat the resistant strains.7
Various factors, including the availability and affordability of medications, healthcare practices, and prescribing patterns, influence the prevalence of anti-fungal use in India.8
Unfortunately, a significant proportion of anti-fungal prescriptions are unnecessary or excessive, contributing to the development of anti-fungal resistance.7 Moreover, there is a lack of data on antifungals’ toxicities and pharmacological interactions, primarily due to limited awareness about anti-fungal usage.9
To address these concerns, an anti-fungal stewardship program (AFSP) is essential to ensure the judicious use of antifungals and prevent the development of anti-fungal resistance.10 A well-structured AFSP can optimise anti-fungal use, minimise the adverse outcomes, and reduce the financial burden of anti-fungal therapy.11 One practical approach for monitoring anti-fungal use is through antimicrobial consumption indicators, such as Days of Therapy (DOT) and Defined Daily Dose (DDD).12 The Centers for Disease Control and Prevention (CDC) and the National Healthcare Safety Network (NHSN) recommend using DOT as the most accurate and preferred measure of antimicrobial use. This study used the DOT metric to analyse anti-fungal consumption due to its advantages over the DDD method.13,14
This study was done to obtain benchmarking data on anti-fungal consumption by DOT metric, to compare the consumption of anti-fungal agents among hospital locations, and to identify target locations for anti-fungal stewardship.
METHODOLOGY
Ethical consideration:
The study was approved by the Institutional Ethics Committee, Bhaikaka University, Karamsad, Anand, and individual consent was waived for this retrospective analysis.
Settings:
The study was conducted at the Microbiology laboratory of Central Diagnostic Laboratory (NABL accredited), Shree Krishna Hospital (NABH accredited), Bhaikaka University, Karamsad, Gujarat.
Data collection:
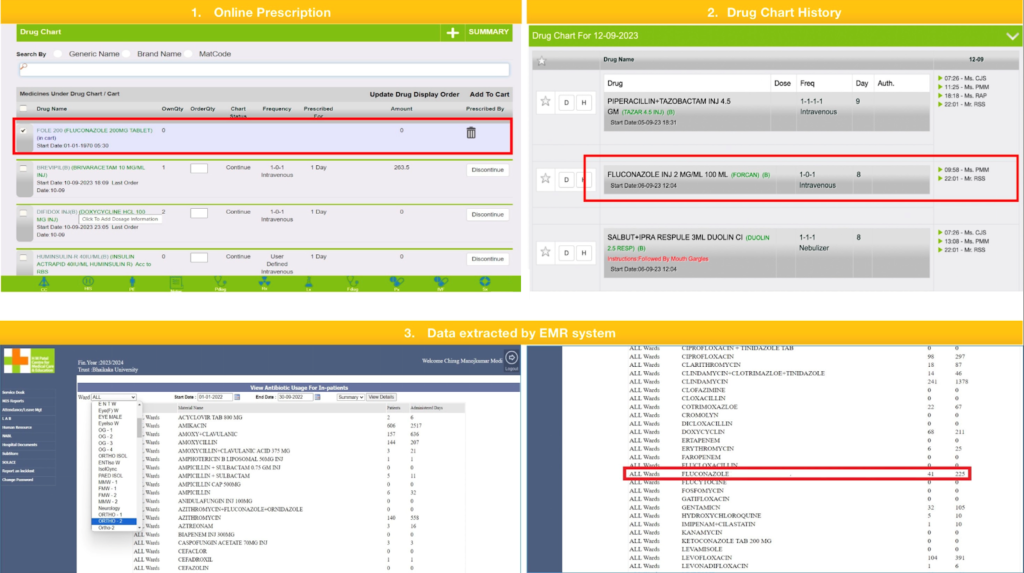
The retrospective observational study was conducted from January 2020 to December 2022 (3 years). All adult in-patients administered with oral or parenteral anti-fungal agents, namely amphotericin B, anidulafungin, caspofungin, fluconazole, flucytosine and voriconazole, were included in the study. All day-care patients, outpatients and all pediatric in-patients to whom topical anti-fungal agents were administered were excluded from this study. Data was captured from the Hospital Information System (HIS) of Shree Krishna Hospital, Bhaikaka University, Karamsad. Our hospital has an Electronic medical record (EMR) system for outpatient and inpatient services, SOLACE, which stands for Shree Krishna Hospital On-Line Application for Clinical Excellence. It contains an online prescription and drug administration chart that captures the drug’s schedule, date, and time of administration. When a drug was prescribed to a patient by a clinician, it showed up on the drug chart history of that patient, and when a nurse administered it, it was captured in a nursing chart. The antifungal agent’s name, number of patients in whom the drug was administered, total administered days, and patient’s hospital number according to timeline and location were extracted by the EMR system (Figure 1). With the help of this, we got the anti-fungal consumption data for the years 2020, 2021 and 2022.
Unit of measurement:
The DOT metric evaluates the days a patient receives an antimicrobial agent (regardless of dose). Any dose of an anti-fungal agent received for 24 hours represented one (1) DOT. The DOT for a given patient on multiple anti-fungal agents was the sum of DOT for each anti-fungal agent the patient received. DOT is often standardised to 1000 Patient Days (DOT/1000 Patient Days) to compare hospitals or services of different sizes.13,14
Figure 1: Online prescription, drug chart history and data extraction by EMR system
Days of therapy with an agent during a period
DOT /1000 PD = _________________________________________________________ X 1000
Total number of Patient Days within that period
Patient days were calculated simultaneously by recording the number of patients at the inpatient location by the HIS. At the end of the month, the sum of all days was recorded electronically. At the same time, we could get the Facility-Wide Inpatient Admission Count and Inpatient Location-Specific Admission Count from HIS.15,16
The following formulas were used for the calculations:
1. | Total consumption of anti-fungal agents = |
Total DOTs of antifungals in last 3 years ______________________________________ X 1000 Total Patient Days for the last 3 years | |
2. | Yearly consumption of anti-fungal agents = |
Total DOTs of anti-fungal agents in a year ______________________________________ X 1000 Total Patient Days for that year | |
3. | Yearly consumption of a particular anti-fungal agent |
Total DOTs of that anti-fungal agent in a year _________________________________________ X 1000 Total Patient Days for that year | |
4. | Yearly use of anti-fungal agents |
No. of patients (anti-fungal agents administered) ___________________________________________ X 1000 Total no. of patients in a year | |
5. | Location-wise use of anti-fungal agents |
No. of patients (anti-fungal agents administered) ____________________________________________ X 100 Total no. of patients in a year at that location |
Data analysis: Data was entered and analysed using Microsoftâ Excel for Mac version 16.75 (23070901).
RESULT
During the study period, 172379 patients were admitted to our hospital. Among them, 183 patients were prescribed anti-fungal agents. Standardising it to 1000 admissions according to formula-4 mentioned above, one patient was prescribed an antifungal agent per 1000 admissions. In the present study, anti-fungal consumption was calculated using a DOT metric. Overall anti-fungal consumption was 2.63, 2.56 and 3.81 DOT/1000 PD, respectively, for 2020, 2021 and 2022 (figure 2).
Figure 2 Pattern of outcomes for de-escalation suggestion made by the antimicrobial stewardship team
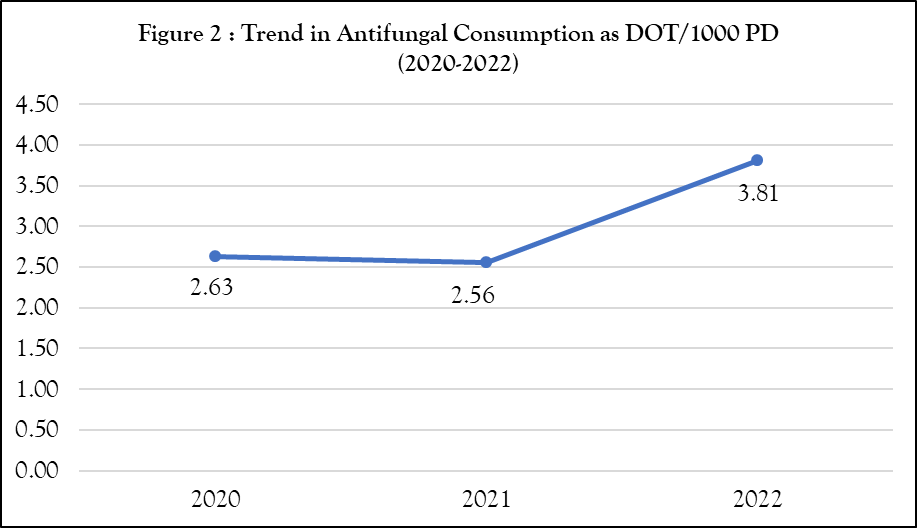
Table 1 shows the yearly consumption of individual anti-fungal agents. Fluconazole was the most consumed anti-fungal agent, having 2.48 DOT/1000 PD. In 2021, amphotericin B, anidulafungin, caspofungin and voriconazole consumption was increased while fluconazole consumption was decreased. In 2022, fluconazole was the most consumed anti-fungal agent (Table 1).
Table 1: Consumption (DOT/1000 PD) of anti-fungal agents (2020-2022) | ||||
Anti-fungal agents | 2020 | 2021 | 2022 | Overal l |
Amphotericin B | 0.00 | 0.16 | 0.01 | 0.06 |
Anidulafungin | 0.00 | 0.03 | 0.00 | 0.01 |
Caspofungin | 0.06 | 0.07 | 0.06 | 0.06 |
Fluconazole | 2.21 | 1.81 | 3.57 | 2.48 |
Flucytosine | 0.00 | 0.00 | 0.00 | 0.00 |
Voriconazole | 0.36 | 0.49 | 0.18 | 0.35 |
Overall | 2.63 | 2.56 | 3.81 | 2.96 |
We also analysed location-wise consumption of antifungals, which showed the Critical Care Unit (CCU) (7.48) having the highest consumption, followed by special wards (5.08) and the medical ward (3.33) (figure 3). Special wards were privileged category wards for patients who wanted to get personalised treatment, including Silver, Gold, Platinum, Deluxe, Super Deluxe and Diamond categories (Figure 3).
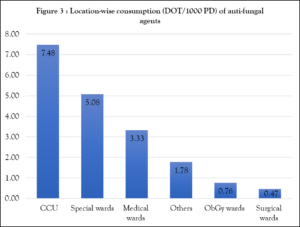
CCU: Critical care unit; Special wards: Silver, Gold, Platinum, Delux, Super Delux, Diamond category wards; Others: ENT, Ophthalmology, Dermatology, Orthopedic, Oncology, Transplantation unit, Burn unit, Emergency care unit; ObGy wards: Obstetrics and Gynaecology wards; Medical & Surgical wards include male & female wards.
In Figure 4, an increasing trend of antifungal consumption was seen in special wards. In 2020 and 2022, CCU (5.46 and 14.01, respectively) had the highest anti-fungal consumption. However, special wards (5.36) had more consumption in 2021 because they were converted to COVID-19-designated wards (figure 4).
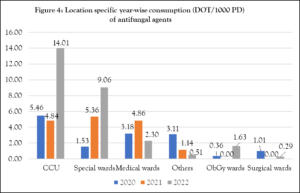 CCU: Critical care unit; Special wards: Silver, Gold, Platinum, Delux, Super Delux, Diamond category wards; Others: ENT, Ophthalmology, Dermatology, Orthopedic, Oncology, Transplantation unit, Burn unit, Emergency care unit; ObGy wards: Obstetrics and Gynaecology wards; Medical & Surgical wards include male & female wards.
CCU: Critical care unit; Special wards: Silver, Gold, Platinum, Delux, Super Delux, Diamond category wards; Others: ENT, Ophthalmology, Dermatology, Orthopedic, Oncology, Transplantation unit, Burn unit, Emergency care unit; ObGy wards: Obstetrics and Gynaecology wards; Medical & Surgical wards include male & female wards.
DISCUSSION
Our study provides valuable insights into anti-fungal consumption using the DOT metric, which has been increasingly recognised as a robust and standardised method for monitoring antimicrobial usage. To the best of our knowledge, no Indian study has been found entirely similar to our study. Our study is the first single-centre study in India demonstrating the versatility of the data on anti-fungal consumption by DOT metric using the EMR system.
In the present study, overall anti-fungal consumption was 2.96 DOT/1000 PD, which was lower than other Indian studies done in 2022 by four single-day point prevalent surveys. In the published study, the overall usage of anti-fungal medications was recorded as 2.71 DDD/1000 PD, with a corresponding figure of 8.96 DOT/1000 PD.17
Figure 2 illustrates the trend in anti-fungal consumption as DOT/1000 PD at Shree Krishna Hospital from 2020 to 2022. A slight decrease was observed in 2021 (2.56 DOT/1000 PD), followed by a significant rise in 2022 (3.81 DOT/1000 PD). This increase may reflect changes in clinical practices, the expanded use of immunosuppressive therapies, or the rise in COVID-19 and mucormycosis cases in India. The prevalence of Mucormycosis in India was 70 times higher compared to global data.18 The observed increase in anti-fungal consumption across the study period also aligns with global trends indicating rising anti-fungal use. A study analysed data from 65 countries reported an increase in systemic anti-fungal consumption from 0.50 to 0.92 defined daily doses (DDD) per 1,000 inhabitants per day between 2008 and 2018.8
Fluconazole’s status as the most commonly consumed anti-fungal agent with 2.48 DOT/1000 PD in our study is consistent with findings from other regions. For example, in Lebanon, fluconazole accounted for 20.99% of total anti-fungal consumption.19 Its widespread use can be attributed to its broad spectrum of activity, favourable pharmacokinetics, and cost-effectiveness. However, the high usage of fluconazole raises concerns about the possible development of resistance in Candida species, which may hamper its effectiveness in treatment.20
The increased use of anti-fungal agents in CCUs (7.48 DOT/1000 PD), as shown in Figure 3, is supported by the published study,21 as critically ill patients are more prone to invasive fungal infections. A multisite study found that echinocandins and fluconazole were CCUs’ most commonly prescribed antifungals, making up 40% and 38% of total consumption, respectively.22 At our institute, special wards were converted to COVID-19-designated wards in 2021, which might be the reason for the increased consumption (5.36 DOT/1000 PD) compared to CCUs (4.84 DOT/1000 PD) in that particular year. In 2022, there was a notable increase in anti-fungal consumption within CCUs (14.01 DOT/1000 PD), possibly due to patients battling both the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and opportunistic fungal infections like mucormycosis, particularly among those with underlying conditions such as diabetes mellitus, cancer, or those receiving hemodialysis treatment combined with deferoxamine. Other probable factors could be disruptions in implementing infection control guidelines, the widespread use of corticosteroids and antibiotics, and increased hospital crowding.23 These circumstances highlight the importance of careful anti-fungal management in CCUs, where patients are especially susceptible to these infections.
In our institution, oncology patients are not exclusively admitted to the oncology ward; they are also placed in medical, surgical, special wards, or CCUs based on their comorbid conditions. This admission practice explains the variation in our findings compared to published studies, where significant anti-fungal consumption was reported in dedicated oncology units.17,24,25
Since the late 1960s, when antifungal compounds were first introduced, resistant fungal strains have been identified in numerous countries worldwide. This has led to a steady rise in invasive infections caused by resistant fungi, becoming an increasing concern for healthcare professionals. The development of triazoles and echinocandins in the 1990s expanded treatment options; however, these advancements were soon followed by the emergence of fungal pathogens resistant to these drugs.26 Azole resistance in Candida and Aspergillus species presents significant clinical challenges alongside echinocandin and multidrug resistance, particularly in Candida glabrata. The spread of azole-resistant Aspergillus fumigatus, often linked to agricultural use, and the growing threat of multidrug-resistant Candida auris is especially concerning.27
In India, infections caused by Candida albicans are relatively common, accounting for 37.95% of cases; however, anti-fungal resistance is more prevalent among non-albicans Candida (NAC) species. Approximately 10.34% of Candida albicans isolates exhibit drug resistance. Resistance to certain azoles, such as fluconazole, is also notable (21.8%.)28 Species like Candida auris pose a significant challenge due to their high resistance to anti-fungal medications and ability to spread easily in healthcare settings.6 Furthermore, 64–71% of multi-triazole-resistant strains have been identified in patients with Aspergillus infections, even those without prior exposure to triazole treatments.29
Prolonged or repeated exposure to anti-fungal drugs accelerates fungal adaptation, leading to resistance. Factors such as low dosages or short treatment durations contribute to the emergence of resistance.6 The increasing use of antifungal agents and the accompanying rise in antifungal resistance highlight the need for Anti-Fungal Stewardship (AFS) programs. These initiatives focus on ensuring the appropriate and judicious use of antifungal agents to improve treatment outcomes.
Monitoring the anti-microbial consumption trend in hospitals will serve as an essential intervention in AMSP, and therefore, hospitals need reliable methods to determine the consumption of antimicrobial agents, including antifungal agents. Antimicrobial consumption data using dispensing and procurement criteria cannot be used for DOT calculation, while prescription records/nursing charts/audits & feedback are suitable for DOT analysis. Nursing chart (administration) data is the most accurate data on antimicrobial usage as it records the data of antimicrobials administered to the patients. Therefore, the present study used nursing chart data to calculate DOT. However, it was cumbersome and impossible to compile nursing chart data without electronically maintained data.30 Published studies agreed that Electronic Health Record Systems (EHRS) were the only way to get accurate data on antimicrobial consumption.31 Canadian acute care pediatric hospital study stated that Electronic Medication Administration Records (eMAR) was a better source of antimicrobial consumption data than pharmacy dispensing systems.32 Our institute has a well-established HIS, and SOLACE was introduced in 2014. Therefore, it is recommended that each institute try to introduce HIS and explore ways of using it for antimicrobial consumption data and various AMSP initiatives.
Our study had various limitations. This being a retrospective study, the rationality of the prescription couldn’t be assessed. Due to the different formats of HIS before 2020, data from more than the last 3 years could not be analysed. The drug chart for pediatric patients was maintained manually due to dose adjustment according to weight; therefore, we could not capture data through SOLACE.
CONCLUSION
Our study showed increased consumption of systemic anti-fungal agents in adult inpatients over three years. A prospective study is recommended to measure anti-fungal consumption trends and assess the rationality of anti-fungal agent prescriptions in locations with high anti-fungal consumption. This study will be a pilot study to enhance the anti-fungal stewardship program.
ACKNOWLEDGEMENT
We thank our Information Technology (Systems) department, particularly Ms. Sejal Shah, for their support in developing a mechanism for calculating antimicrobial consumption data through our HIS.
SOURCE OF FUNDING
None
CONFLICTS OF INTEREST STATEMENT
The authors declare no conflict of interest.
AUTHOR’S CONTRIBUTION
RP: Conceptualization; Methodology; Resources; Data collection; Analysis; Writing the draft
BC: Data collection; Literature review; Analysis; Writing the draft; Review & Editing
CM: Conceptualization; Resources; Data collection; Analysis; Writing the draft; Review & Editing
NP: Conceptualization; Resources; Data collection; Analysis; Writing the draft; Review & Editing
REFERENCES
Ray A, Adarsh Aayilliath K, Banerjee S, Chakrabarti A, Denning DW. Burden of Serious Fungal Infections in India. Open Forum Infect Dis. 2022;9(12).
Bongomin F, Gago S, Oladele RO, Denning DW. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J Fungi (Basel). 2017;3(4):57.
Worldometer. India Population (LIVE). Accessed December 20, 2024. https://www.worldometers.info/world-population/india-population/
The University of Manchester. Study reveals huge extent of fungal disease in India. Published January 3, 2023. Accessed December 20, 2024. https://www.manchester.ac.uk/about/news/study-reveals-huge-extent-of-fungal-disease-in-india/
Chakrabarti A, Slavin MA. Endemic fungal infections in the Asia-Pacific region. Med Mycol. 2011;49(4):337-44.
Hossain CM, Ryan LK, Gera M, et al. Antifungals and Drug Resistance. Encyclopedia. 2022;2(4):1722-37.
CDC. Antimicrobial-Resistant Fungal Diseases. Atlanta: United States Center for Disease Control and Prevention. Accessed December 20, 2024. https://www.cdc.gov/fungal/antimicrobial-resistant-fungi/index.html
Pathadka S, Yan VKC, Neoh CF, et al. Global Consumption Trend of Antifungal Agents in Humans From 2008 to 2018: Data From 65 Middle- and High-Income Countries. Drugs. 2022;82(11):1193-1205.
Ghannoum MA, Rice LB. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev. 1999;12(4):501-17.
Ray A, Das A, Panda S. Antifungal stewardship: What we need to know. Indian J Dermatol Venereol Leprol. 2023;89(1):5-11.
Albahar F, Alhamad H, Abu Assab M, Abu-Farha R, Alawi L, Khaleel S. The Impact of Antifungal Stewardship on Clinical and Performance Measures: A Global Systematic Review. Trop Med Infect Dis. 2023;9(1):8.
Vallès J, Fernández S, Cortés E, et al. Comparison of the defined daily dose and days of treatment methods for evaluating the consumption of antibiotics and antifungals in the intensive care unit. Med Intensiva. 2020;44(5):294-300.
Public Health Ontario. Antimicrobial Stewardship Programs (ASPs) Metrics Examples. Accessed December 20, 2024. https://www.publichealthontario.ca/-/media/documents/A/2017/asp-metrics-examples.pdf.
WHO. Antimicrobial stewardship programmes in health-care facilities in low- and middle-income countries: a WHO practical toolkit. Geneva: World Health Organization. Accessed December 20, 2024. https://www.who.int/publications/i/item/9789241515481
CDC. Patient Days. Atlanta: United States Center for Disease Control and Prevention. Accessed December 20, 2024. https://nhsn.cdc.gov/nhsntraining/courses/da-intro/index.html?jmptopg=page336.html#top
CDC. Determining Patient Days for Summary Data Collection: Observation vs. Inpatients. Atlanta: United States Center for Disease Control and Prevention. Accessed December 20, 2024. https://www.cdc.gov/nhsn/pdfs/commup/patientday_sumdata_guide.pdf
Kaur H, Krishnamoorthi S, Dhaliwal N, et al. Antifungal prescription practices and consumption in a tertiary care hospital of a developing country. Mycoses. 2022;65(10):935-45.
Prakash H, Chakrabarti A. Epidemiology of Mucormycosis in India. Microorganisms. 2021;9(3):523.
Rahme D, Ayoub M, Shaito K, Saleh N, Assaf S, Lahoud N. First trend analysis of antifungals consumption in Lebanon using the World Health Organization collaborating center for drug statistics methodology. BMC Infect Dis. 2022;22(1):882.
Fisher MC, Alastruey-Izquierdo A, Berman J, et al. Tackling the emerging threat of antifungal resistance to human health. Nat Rev Microbiol. 2022;20(9):557-71.
Fondevilla E, Grau S, Mojal S, et al. Consumption of systemic antifungal agents among acute care hospitals in Catalonia (Spain), 2008-2013. Expert Rev Anti Infect Ther. 2016;14(1):137-44.
Logan C, Hemsley C, Fife A, et al. A multisite evaluation of antifungal use in critical care: implications for antifungal stewardship. JAC Antimicrob Resist. 2022;4(3):dlac055.
Abd El-Baky RM, Shady ER, Yahia R, et al. COVID-19 associated Mucormycosis among ICU patients: risk factors, control, and challenges. AMB Express. 2023;13(1):99.
Marins TA, Marra AR, Edmond MB, et al. Evaluation of Candida bloodstream infection and antifungal utilization in a tertiary care hospital. BMC Infect Dis. 2018;18(1):187.
Awad L, Tamim H, Abdallah D, et al. Correlation between antifungal consumption and the distribution of Candida species in different hospital departments of a Lebanese medical Centre. BMC Infect Dis. 2018;18(1):589.
Hokken MWJ, Zwaan BJ, Melchers WJG, Verweij PE. Facilitators of adaptation and antifungal resistance mechanisms in clinically relevant fungi. Fungal Genet Biol. 2019;132:103254.
Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect Dis. 2017;17(12):e383-92.
Verma R, Pradhan D, Hasan Z, Singh H, Jain AK, Khan LA. A systematic review on distribution and antifungal resistance pattern of Candida species in the Indian population. Med Mycol. 2021;59(12):1145-65.
Hoda S, Agarwal H, Ahluwalia Simran K, Vermani M, Vijayaraghavan P. Antifungal Resistance Analysis of environmental isolates of Aspergillus in North India. J Pure Appl Microbiol. 2019;13:385-92.
Sastry A, Priyadarshi K, Sarumathi D. Essentials of Antimicrobial Stewardship. Jaypee Brothers Medical Publishers; 2023.
Rezel-Potts E, Gulliford M. Electronic Health Records and Antimicrobial Stewardship Research: a Narrative Review. Curr Epidemiol Rep. Published online July 21, 2022. doi:10.1007/s40471-021-00278-1
Dalton BR, MacTavish SJ, Bresee LC, et al. Antimicrobial use over a four-year period using days of therapy measurement at a Canadian pediatric acute care hospital. Can J Infect Dis Med Microbiol. 2015;26(5):253-8.
Submit a Manuscript:
Copyright © Author(s) 2024. JASPI- Journal of Antimicrobial Stewardship Practices and Infectious Diseases.

