Efficacy of a Customized Holistic Traditional Complementary Alternative Medicine Protocol for Improving Immunological, Metabolic, and Clinical Parameters in Mild to Moderate COVID-19 Patients: A Pilot Randomized Controlled Trial
JASPI December 2024/ Volume 2/Issue 4
Copyright: © Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Mirza AA, Gaur A, Kalyani CV, et al.Efficacy of a Customized Holistic Traditional Complementary Alternative Medicine Protocol for Improving Immunological, Metabolic, and Clinical Parameters in Mild to Moderate COVID-19 Patients: A Pilot Randomized Controlled Trial. JASPI. 2024;2(4):25-31 DOI: 10.62541/jaspi051
ABSTRACT
Background: The COVID-19 pandemic has necessitated exploring various treatment options, including Traditional Complementary and Alternative Medicine. This study aims to evaluate the efficacy of a customised Holistic Traditional Complementary Alternative Medicine (HTCAM) protocol in improving immunological, metabolic, and inflammatory outcomes in patients with mild to moderate COVID-19.
Methods: This pilot randomised controlled trial involved 100 patients, 50 in the HTCAM intervention group and 50 in the control group. Participants were adults diagnosed with mild to moderate COVID-19. Following 1:1 randomisation, baseline data were collected, including blood samples for interleukins, oxidative stress, and metabolic parameters. The HTCAM group received a detailed, customised protocol with daily supervision and clinical monitoring. Blood samples were collected at discharge or on the 7th day post-enrollment. Statistical analysis was performed using SPSS version 25.0, employing mean, standard deviation, chi-square tests, and independent t-tests, with significance set at p < 0.05.
Results: Sociodemographic and clinical variables showed no significant differences between groups. Significant improvements in the HTCAM group were observed in creatinine and triglyceride levels compared to the control group. Significant differences were also noted in serum albumin and TNF-alpha levels, enhancing inflammation management and overall health.
Conclusion: This pilot trial suggests combining the Holistic Traditional Complementary Alternative Medicine (HTCAM) protocol with conventional COVID-19 treatment may improve clinical and metabolic parameters. Significant benefits were observed in serum albumin levels and reductions in TNF-alpha, with HTCAM also maintaining stable ferritin levels and positively influencing triglycerides and creatinine. Further research must confirm these findings and clarify HTCAM’s role in COVID-19 management.
KEYWORDS: Holistic medicine; complementary therapies; immunological; metabolic; COVID-19
INTRODUCTION
The COVID-19 pandemic, caused by the SARS-CoV-2 virus, has profoundly impacted global health systems, highlighting the need for effective treatment options. The disease’s severe respiratory manifestations and variable progression have led to an extensive search for both conventional and alternative therapeutic approaches. Among these, Traditional Complementary and Alternative Medicine (TCAM) has garnered increasing attention for its potential role in managing COVID-19.1,2
TCAM encompasses diverse practices, including herbal medicine, acupuncture, and dietary interventions, rooted in longstanding cultural traditions. Recent reviews suggest that TCAM approaches, with their holistic principles and multi-faceted mechanisms, may offer complementary benefits in treating COVID-19.3,4 For instance, herbal formulations used in traditional Chinese medicine have been shown to modulate inflammatory responses and enhance immune function, potentially addressing some of the pathological features of COVID-19.5,6 Similarly, dietary interventions and mind-body practices are recognised for supporting immune health and improving overall well-being, which could be beneficial in the context of a pandemic.7,8
Despite these promising findings, rigorous clinical evaluations of TCAM in the context of COVID-19 are still limited. Most existing studies are observational or anecdotal, with insufficient data from randomised controlled trials to substantiate the efficacy of these approaches in COVID-19 management.9,10 The lack of robust evidence underscores the need for well-designed clinical trials to evaluate the safety and effectiveness of TCAM therapies.
This study aims to address this gap by investigating the impact of a Holistic Traditional Complementary Alternative Medicine (HTCAM) protocol in patients with mild to moderate COVID-19. By comparing the outcomes of patients receiving HTCAM with those receiving standard conventional care, this trial seeks to provide empirical data on the potential benefits of integrating TCAM approaches into traditional treatment regimens for COVID-19. The primary objectives are to assess improvements in immunological, while these secondary objectives are clinical and metabolic parameters and to evaluate any associated changes in patient outcomes.
METHODOLOGY
Study design and participants:
This pilot randomised controlled trial included 100 participants, with 50 in the intervention group and 50 in the control group. All participants were diagnosed with mild to moderate SARS-CoV-2 infection (As per guidelines of ‘clinical management protocol: COVID-19’ laid by the Government of India – Ministry of Health and Family Welfare Directorate General of Health Services (EMR Division)) and were receiving treatment at the tertiary care centre during 2020-21.11
Inclusion and exclusion criteria:
The inclusion criteria were mild to moderate COVID-19 participants aged 18 years, able and willing to participate, and had not taken any herbs or complementary therapies in the past 8 weeks. Exclusion criteria included enrollment in other COVID-19 prevention or treatment trials, allergies or sensitivities to food items, comorbidities such as metabolic, psychiatric, neurological, or autoimmune disorders, secondary malignancy, steroid therapy, significant physical debility, and pregnancy.
Randomisation and group allocation:
Participants were randomised using a block randomisation technique, with a 1:1 allocation ratio using a computer-generated number using the SNOOSE method between the intervention and control groups.
Control group:
The control group comprised 50 participants. Baseline data were collected through interviews, and blood samples were drawn, labelled, transported, and analysed on the same day for COVID-19. Samples were also stored for later analysis of interleukins, oxidative stress, and antioxidant parameters.
Intervention group:
The intervention group, consisting of 50 participants, received Holistic Traditional Complementary Alternative Medicine (HTCAM) treatment (details of which are attached in Supplementary file 1). These participants followed a customised HTCAM protocol under daily supervision, with clinical signs, symptoms, and blood samples monitored at discharge or on the 7th-day post-enrollment.
Outcomes:
The primary outcomes of the study are (kidney function, liver function, lipid, and other metabolic indices), and the secondary outcomes are Inflammatory and oxidative stress markers.
Statistical Analysis:
Statistical analyses were conducted using SPSS version 25.0. Descriptive statistics (mean and SD) were used, and inferential statistics (independent sample t-tests) were employed to assess group differences. A p-value of <0.05 was considered statistically significant.
Ethical Considerations:
Ethical approval was obtained from the Institute Ethical Committee. Written informed consent was obtained from each participant before inclusion in the study.
RESULTS
Baseline characteristics:
The study included 100 participants, 50 in the intervention group and 50 in the control group (Figure 1). The mean age in both groups was comparable. Gender distribution showed more males in the control group, but this was not statistically significant. Educational and occupational statuses, as well as marital status, were similar across both groups. The detailed baseline characteristics are summarised in Table 1.
Figure 1: CONSORT Flow.
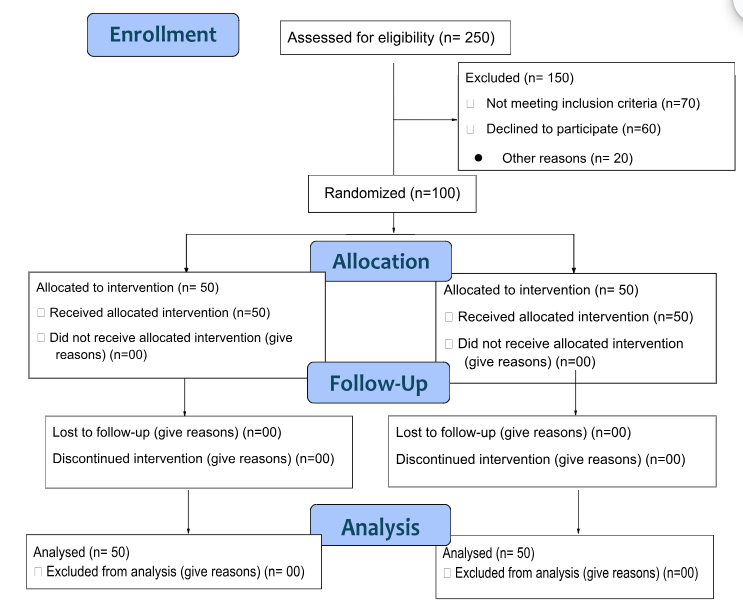
Table 1: Socio-demographic and clinical characteristics among two groups.
|
S. |
Variable |
Sub-categories |
Groups f (%) |
p- |
|
|---|---|---|---|---|---|
|
Experimental group (n=50) |
Control group (n=50) |
||||
|
1 |
Age |
Mean (±SD) |
35.04 ± 11.79 |
36.19 ± 9.52 |
0.001* |
|
2 |
Gender |
Male |
26 (52) |
35 (70) |
0.065 |
|
3 |
Educational status |
Graduate and Higher |
44 (88) |
45 (90) |
0.842 |
|
4 |
Occupational status |
Service |
35 (70) |
35 (70) |
0.442 |
|
Student |
09 (18) |
13 (26) |
|||
|
Others |
06 (12) |
02 (04) |
|||
|
5 |
Marital status |
Married |
29 (58) |
26 (52) |
0.546 |
|
6 |
Body Mass Index |
Mean ±SD |
21.47± 3.33 |
21.79± 1.75 |
0.555 |
|
7 |
Dietary pattern |
Vegetarian |
22 (44) |
20 (40) |
0.685 |
|
8 |
Alcohol intake |
NO |
47 (94) |
47 (94) |
1.000 |
|
9 |
Fever |
Yes |
42 (84) |
41 (82) |
0.790 |
|
10 |
Cough |
Yes |
36 (72) |
35 (70) |
0.826 |
|
11 |
Body Pain |
Yes |
36 (72) |
35 (70) |
0.826 |
|
12 |
Nausea |
Yes |
9 (18) |
9 (18) |
1.000 |
|
13 |
Diarrhea |
Yes |
18 (36) |
18 (36) |
1.000 |
*p value <0.05 represents significance; Chi-square is used to assess the significance of categorical data; for continuous data (Mean ± SD), an independent sample t-test is used.
Primary outcomes:
The primary outcomes focused on kidney function, lipid profile, liver function, and inflammatory markers. Creatinine levels and triglycerides showed significant differences, favouring the intervention group. Serum albumin was also significantly higher in the intervention group, while TNF-alpha levels were reduced compared to the control group. Other markers, such as sodium, potassium, and cholesterol, did not differ significantly. Table 2 presents the detailed primary outcomes.
Table 2: Comparison of primary outcomes (kidney function, liver function, lipid, and other metabolic indices) from baseline to end of the trial between experimental and control groups.
|
S. No. |
Variables |
Difference in Experimental group (n= 50) |
Difference in Control group (n=50) |
p-value |
|
1 |
Sodium |
-1.15 (-35 to 34) |
-0.35 (-100.4 to 16) |
0.393 |
|
2 |
Potassium |
-0.125 (-1.97 to 3.02) |
-0.16 (-1.79 to 1.16) |
0.962 |
|
3 |
Urea |
-1.85(-38.3 to 32.5) |
1.0 (-61.5 to 26.3) |
0.279 |
|
4 |
Creatinine |
-0.09 (-0.8 to 3.89) |
0.015 (-6.73 to 1.97) |
0.003* |
|
5 |
Chloride |
-0.15 (-49 to 24.10) |
0.15 (-94.47 to 13) |
0.398 |
|
6 |
GGT |
-0.1 (-28.5 to 66) |
-4.8 (-273.8 to 224.1) |
0.122 |
|
7 |
Total Bilirubin |
-0.05(-0.45 to 0.71) |
-0.02 (-0.8 to 1.34) |
0.586 |
|
8 |
Direct Bilirubin |
-0.04(-0.44 to 0.17) |
-0.05 (-0.54 to 0.84) |
0.929 |
|
9 |
Total protein |
0.0(-3.46 to 2.88) |
0.16(-2.02 to 3.14) |
0.145 |
|
10 |
Albumin |
-0.02(-0.87 to 1.0) |
0.26(-0.87 to 3.78) |
0.01* |
|
11 |
ALP |
-5.25(-20.2 to155.2) |
-7.2(-196.9 to 474) |
0.877 |
|
12 |
AST |
-1.05(-51.8 to 29.5) |
3.65(-196.5 to 80) |
0.486 |
|
13 |
Triglycerides |
-13.4 (-418.80 to 128) |
7.2 (-509.5 to 154.2) |
0.020* |
|
14 |
Total Cholesterol |
-4.00 (-182 to 59) |
-12.15 (-124.5 to 98.3) |
0.484 |
|
15 |
LDL-C |
-8.35 (-131.2 to 72) |
-11.15 (-125 to 55) |
0.697 |
|
16 |
HDL-C |
-4.525 (-46 to 76.4) |
0.90 (-36.2 to 41.4) |
0.372 |
|
17 |
Calcium |
0.145 (-3.26 to 4.69) |
0.155 (-6.7 to 2.94) |
0.866 |
|
18 |
Phosphorus |
0.19(-3.49 to 5.78) |
0.60 (-7.52 to 9.15) |
0.151 |
|
19 |
Amylase |
-1.05(-62.4 to 267) |
-1.1 (102 to 109.8) |
0.699 |
|
20 |
T3 |
0.19 (-1.02 to 1.92) |
0.19 (-1.31 to 2.68) |
0.515 |
|
21 |
T4 |
0.01 (-0.27 to 0.72) |
0.06 (-1.28 to 1.13) |
0.296 |
|
22 |
TSH |
0.34 (-6.14 to 7.26) |
0.00 (-9.23 to 83.07) |
0.145 |
*p value <0.05 represents significance; Values in Median (IQR), Mann-Whitney U test used for assessing difference;
Abbreviations: GGT: Gamma-Glutamyl Transferase ALP: Alkaline Phosphatase AST: Aspartate Aminotransferase LDL-C: Low-Density Lipoprotein Cholesterol HDL-C: High-Density Lipoprotein Cholesterol T3: Triiodothyronine T4: Thyroxine TSH: Thyroid-Stimulating Hormone
Secondary outcomes: The secondary outcomes included oxidative stress markers, bone-related markers, and thyroid profiles. No significant differences were found between the groups in these areas. Table 3 provides the details of the secondary outcomes.
Table 3: Changes in Inflammatory and oxidative stress markers from baseline to end of the trial between the Experimental group and Control group.
|
S. N. |
Variables |
Differences in Experimental group (n= 50) |
Differences in Control group (n=50) |
p-value |
|
1 |
IL-6 |
-4.5800 (-5499.99 to 4899.00) |
-.0150 (-5499.99 to 4898.50) |
0.728 |
|
2 |
IL-10 |
0.0 (-234.85 to 15.0) |
0.0 (-234.85 to 15.00) |
0.653 |
|
3 |
TNF alpha |
-0.05 (-411.00 to 123.20) |
-31.45 (-558.30 to 123.2) |
0.05* |
|
4 |
CRP |
-11.015 (-158.50 to 4.79) |
-21.555 (-148.50 to 4.79) |
0.519 |
|
5 |
Pro-calcitonin |
0 (-5.69 to 0.04) |
0 (-0.19 to 0.51) |
0.466 |
|
6 |
Iron |
-0.35 (-121.1 to 132.7) |
-7.3 (-204.2 to 176.9) |
0.254 |
|
7 |
Ferritin |
-0.46 (-378.05 to 790.24) |
-15.63 (-320.66 to 219.9) |
0.106 |
|
8 |
MDA |
-0.0327 (-.32 to 0.32) |
-0.03(-0.12 to -0.02) |
0.842 |
|
9 |
TAS |
-129.165 (-2123.16 to 678.27) |
-133.7873 (-954.54 to 392.65) |
0.901 |
|
10 |
SOD |
5.05(-98.7 to 78.4) |
9.75 (-58 to 89.9) |
0.340 |
*p value <0.05 represent significance; Values in Median (IQR), Mann-Whitney U test used for assessing difference; Abbreviations: IL-6: Interleukin 6 IL-10: Interleukin 10 TNF-α: Tumor Necrosis Factor-alpha CRP: C-Reactive Protein MDA: Malondialdehyde TAS: Total Antioxidant Status SOD: Superoxide Dismutase.
DISCUSSION
The COVID-19 pandemic has highlighted the necessity for diverse and effective treatment strategies. As SARS-CoV-2 continues to impact global health, integrating complementary approaches such as Holistic Traditional Complementary Alternative Medicine (HTCAM) into conventional treatment regimens has garnered interest. This study’s findings highlight the potential benefits of HTCAM in managing COVID-19, particularly in improving serum albumin levels and modulating inflammatory markers.
Our study observed significant changes in serum albumin and TNF-alpha levels among patients receiving HTCAM compared to those on standard allopathic treatments. Elevated serum albumin levels have been associated with better clinical outcomes in COVID-19 patients, as low levels often indicate poor prognosis and increased mortality. Improving serum albumin among patients receiving HTCAM supports its potential role in enhancing patient recovery and managing inflammation. This finding is consistent with recent research suggesting that maintaining or increasing serum albumin levels can improve immune function and reduce the risk of severe outcomes in COVID-19 patients.12
The observed reduction in TNF-alpha levels is noteworthy, as this pro-inflammatory cytokine is known to contribute to disease severity in COVID-19. Elevated TNF-alpha levels have been linked to hyperinflammation and severe clinical outcomes, making its modulation a critical aspect of effective treatment. HTCAM’s impact on TNF-alpha suggests it may help reduce inflammation and associated complications. This finding aligns with studies showing the anti-inflammatory effects of various complementary therapies, including herbal medicines and dietary supplements, in managing cytokine storm and systemic inflammation in COVID-19 patients.13,14
In contrast, the study found that serum ferritin levels were adversely affected by standard treatments, whereas HTCAM showed no significant change. Elevated ferritin levels are often associated with inflammation and liver dysfunction in COVID-19. The ability of HTCAM to maintain stable ferritin levels could indicate its role in managing systemic inflammation and preventing complications related to ferritin dysregulation. This is consistent with research suggesting that specific complementary therapies can modulate ferritin levels and improve disease management.15,16
Additionally, the study’s findings on lipid profiles and creatinine levels further underscore the potential benefits of HTCAM. Hypertriglyceridemia and elevated creatinine levels have been observed in COVID-19 patients, and managing these parameters is crucial for comprehensive care.17,18 HTCAM’s positive effect on these markers suggests its utility in addressing metabolic and renal concerns associated with the disease. Previous studies have highlighted the role of dietary and herbal interventions in improving lipid metabolism and kidney function, which could contribute to better overall outcomes in COVID-19 patients.19
Moreover, integrating HTCAM with conventional therapies might enhance overall patient well-being and reduce the burden on healthcare systems. The holistic nature of HTCAM, which often includes dietary advice, lifestyle modifications, and stress management techniques, could be supportive in managing long-term COVID-19 symptoms and recovery. This approach aligns with the growing recognition of the importance of holistic and patient-centred care in managing chronic and complex diseases.
In our study, we recognise several limitations that impact the findings. Firstly, the statistically significant age difference between the two groups constitutes a known confounder, potentially influencing the outcomes. Furthermore, conducting a comprehensive baseline comparison of primary outcomes—such as kidney function, liver function, lipid levels, and other metabolic indices—would have enhanced our understanding of the initial comparability between the groups.
Overall, our findings contribute to the evolving evidence on the potential benefits of incorporating complementary approaches into conventional COVID-19 treatment regimens. While our study provides promising insights, further research with larger sample sizes and rigorous methodologies is essential to validate these results and establish comprehensive guidelines for integrating HTCAM into standard treatment protocols. Future studies should also explore the mechanisms underlying the observed effects and assess the long-term outcomes of HTCAM interventions in diverse patient populations. Despite these promising findings, integrating HTCAM into standard treatment regimens requires further investigation to establish its efficacy and safety comprehensively.
CONCLUSION
This pilot randomised controlled trial demonstrates that the Holistic Traditional Complementary Alternative Medicine (HTCAM) protocol may offer benefits when combined with conventional treatment for COVID-19. The study observed significant improvements in serum albumin levels and reductions in inflammatory markers like TNF-alpha among patients receiving HTCAM. Additionally, HTCAM did not adversely affect ferritin levels, negatively impacted by standard treatments. The protocol also showed potential in managing metabolic markers, such as triglycerides and creatinine. While these findings are promising, further research with larger sample sizes is needed to validate these results and fully understand the role of HTCAM in COVID-19 management.
ACKNOWLEDGEMENT
The authors gratefully acknowledge the doctors, COVID-19 patients, and caregivers who helped make the study possible.
CONFLICTS OF INTEREST STATEMENT
The authors declare no conflict of interest.
SOURCE OF FUNDING
The project has been supported financially by the Department of Science and Technology (DST SATYAM Special COVID-19 Call).
AUTHOR’S CONTRIBUTION
AAM: Conceptualization; Data curation; Resources; Methodology; Analysis; Writing the draft; Review & Editing
NG: Conceptualization; Supervision
CVK: Conceptualization; Supervision
KKR: Conceptualization; Supervision
BG: Conceptualization; Supervision
MJ: Conceptualization; Supervision
ASB: Conceptualization; Data curation; Supervision
PKP: Conceptualization; Supervision
SRY: Conceptualization; Data curation; Supervision
RG: Conceptualization; Supervision
SM: Conceptualization; Data curation; Methodology; Supervision
REFERENCES
Kim TH, Kang JW, Jeon SR, Ang L, Lee HW, Lee MS. Use of Traditional, Complementary and Integrative Medicine During the COVID-19 Pandemic: A Systematic Review and Meta-Analysis. Front Med (Lausanne). 2022;9:884573.
Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924.
Wang W, Tang J, Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol. 2020;92(4):441-7.
Wang Y, Tian H, Zhang L, et al. Reduction of secondary transmission of SARS-CoV-2 in households by face mask use, disinfection and social distancing: a cohort study in Beijing, China. BMJ Glob Health. 2020;5(5):e002794.
Zhu Z, Lian X, Su X, Wu W, Marraro GA, Zeng Y. From SARS and MERS to COVID-19: a brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir Res. 2020;21(1):224.
Sharma R, Kumar M, Rohilla KK. COVID-19 Infodemic: Evaluating Information-Seeking Behaviour Among Healthcare Workers During a Pandemic. Cureus. 2022;14(1):e20910.
V’kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19(3):155-70.
Al-Swiahb JN, Motiwala MA. Upper respiratory tract and otolaryngological manifestations of coronavirus disease 2019 (COVID-19): A systemic review. SAGE Open Med. 2021;9:20503121211016965.
Kumar M, Rani P, Joshi B, Soni RK, Kumari A, Rohilla KK. Telemedicine as an unexpected catalyst during and beyond the COVID-19 Pandemic. Nepal J Epidemiol. 2022;12(1):1171-4.
Kermali M, Khalsa RK, Pillai K, Ismail Z, Harky A. The role of biomarkers in diagnosis of COVID-19 – A systematic review. Life Sci. 2020;254:117788.
MoHFW. Clinical management protocol: COVID-19. New Delhi: Ministry of Health and Family Welfare, Government of India. Accessed December 31, 2024. https://covid19dashboard.mohfw.gov.in/pdf/ClinicalManagementProtocolforCOVID19dated27062020.pdf
Xu Y, Yang H, Wang J, Li X, Xue C, Niu C, Liao P. Serum albumin levels are a predictor of COVID-19 patient prognosis: Evidence from a single cohort in Chongqing, China. International journal of general medicine. 2021 Jun 24:2785-97.
Smail SW, Babaei E, Amin K, Abdulahad WH. Serum IL-23, IL-10, and TNF-α predict in-hospital mortality in COVID-19 patients. Frontiers in Immunology. 2023 May 22;14:1145840.
Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, Lavin Y, Swartz TH, Madduri D, Stock A, Marron TU. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nature medicine. 2020 Oct;26(10):1636-43.
Mahroum N, Alghory A, Kiyak Z, Alwani A, Seida R, Alrais M, Shoenfeld Y. Ferritin–from iron, through inflammation and autoimmunity, to COVID-19. Journal of autoimmunity. 2022 Jan 1;126:102778.
Kaushal K, Kaur H, Sarma P, Bhattacharyya A, Sharma DJ, Prajapat M, Pathak M, Kothari A, Kumar S, Rana S, Kaur M. Serum ferritin as a predictive biomarker in COVID-19. A systematic review, meta-analysis and meta-regression analysis. Journal of critical care. 2022 Feb 1;67:172-81.
Kaddoura R, Mohamed Ibrahim MI, Al-Amri M, Prabhakaran Nair A, Alharafsheh A, Alyafei SA, Albakri M. COVID-19-associated hypertriglyceridemia and impact of treatment. Frontiers in Medicine. 2024 Feb 21;11:1326156.
Dai W, Lund H, Chen Y, Zhang J, Osinski K, Jones SZ, Kreuziger LB, López JA, Benjamin IJ, Silverstein RL, Zheng Z. Hypertriglyceridemia during hospitalization independently associates with mortality in patients with COVID-19. Journal of Clinical Lipidology. 2021 Sep 1;15(5):724-31.
Mullin GE, Limektkai B, Wang L, Hanaway P, Marks L, Giovannucci E. Dietary supplements for COVID-19. Coronavirus Disease-COVID-19. 2021 May 11:499-515.
Submit a Manuscript:
Copyright © Author(s) 2024. JASPI- Journal of Antimicrobial Stewardship Practices and Infectious Diseases.

