Carbapenem-resistant Bacteremia and Metastatic Abscesses – A Community-acquired Infection of Concern
JASPI December 2024/ Volume 2/Issue 4
Copyright: © Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Kumar A, Akhtar M, Panda PK.Carbapenem-resistant Bacteremia and Metastatic Abscesses – A Community-acquired Infection of Concern. JASPI. 2024;2(4): 53-58
DOI: 10.62541/jaspi055
ABSTRACT
Systemic dissemination of uropathogens from the genitourinary tract resulting in the formation of psoas and chest wall abscesses represents a rare yet significant complication of urinary tract infections (UTIs). This phenomenon is widespread in high-risk individuals like those with poorly controlled diabetes mellitus, and if left untreated, can lead to severe, potentially fatal outcomes. The community is increasingly concerned about the infections caused by multidrug-resistant pathogens. Here, a man in his 50s with uncontrolled type 2 diabetes (HbA1c = 16.6%) presented with fever, chills, rigours, and right flank pain worsening with hip extension, occasionally radiating to the groin. He developed a painful swelling on the right upper chest with erythema. Diagnostic workup revealed metastatic infection by a carbapenem-resistant Escherichia coli, which began as bilateral pyelonephritis and progressed to bacteremia, psoas abscess, and thoracic wall abscess. All abscesses were resolved with amikacin treatment, which led to a favourable outcome. This case highlights the atypical presentations of carbapenem-resistant Escherichia coli pyelonephritis as well as the diagnostic challenge of atypical Carbapenem-resistant Enterobacterales [CRE] infections in high-risk diabetic patients, emphasising the need for blood and pus cultures to identify multi-drug resistant (MDR) pathogens and guide effective treatment, and prevent antimicrobial resistance (AMR), alongside community education and individualised care for prevention and early intervention
KEYWORDS: Chest wall; Diabetes; Escherichia coli; Hepatitis C; Multidrug-resistant; UTI
BACKGROUND
Community-acquired infections due to MDR Escherichia coli are difficult to diagnose and manage, particularly when such infections present with dual metastatic abscesses, such as psoas and thoracic wall abscesses. UTIs are most commonly caused by E. coli. The increasing prevalence of carbapenem-resistant E. coli strains poses serious concerns, as these pathogens are resistant to standard antibiotics commonly used in their treatment. The clinical manifestation of dual metastatic abscesses resulting from carbapenem-resistant E. coli infections is rare and poorly documented in the existing literature, highlighting a significant gap in understanding such cases’ pathophysiology, diagnosis, and management.1,2
Metastatic abscesses, particularly psoas and chest wall abscesses, are uncommon complications of hematogenous spread of E. coli from a primary uropathogenic source. Although psoas abscesses are more frequently caused by tuberculosis [TB] infection, they can also arise from E. coli, particularly in high-risk individuals with poorly controlled diabetes or those immunocompromised. The rarity and insidious onset of psoas abscesses often lead to delays in its diagnosis, which can delay the treatment and worsen patient outcomes. Similarly, primary chest wall abscesses, though commonly caused by Mycobacterium tuberculosis, have been occasionally reported due to E. coli infections. However, cases caused by carbapenem-resistant E. coli are still underreported in the literature.3
The diagnosis of these dual metastatic abscesses are becoming more challenging due to the subtle, non-specific symptoms, which can be easily overlooked, especially in patients with multiple comorbidities. Furthermore, the emergence of carbapenem-resistant E. coli strains complicates their effective treatment. These pathogens are resistant to various antibiotic classes, reducing the efficacy of standard therapies. Carbapenem antibiotics, once essential in treating UTIs caused by E. coli, are now becoming ineffective due to the spread of carbapenem-hydrolyzing beta-lactamases. This trend makes timely and effective management more complex, requiring alternative therapeutic strategies.4 CRE isolates are often susceptible to at least one aminoglycoside.5 These agents can be used as monotherapy for susceptible CRЕ UTIs. Still, they should not be used as single agents for infections outside the urinary tract due to the lack of supportive clinical data in these settings.6
This case highlights the urgent need to address the knowledge gap regarding dual metastatic abscesses caused by carbapenem-resistant E. coli. Early recognition, comprehensive diagnostic workup—including blood and abscess cultures and radiological imaging for psoas abscess—and the development of effective targeted treatment regimens are essential for improving patient outcomes and preventing AMR. Understanding these rare but serious complications will be critical in effectively managing community-acquired MDR E. coli infections.
CASE PRESENTATION
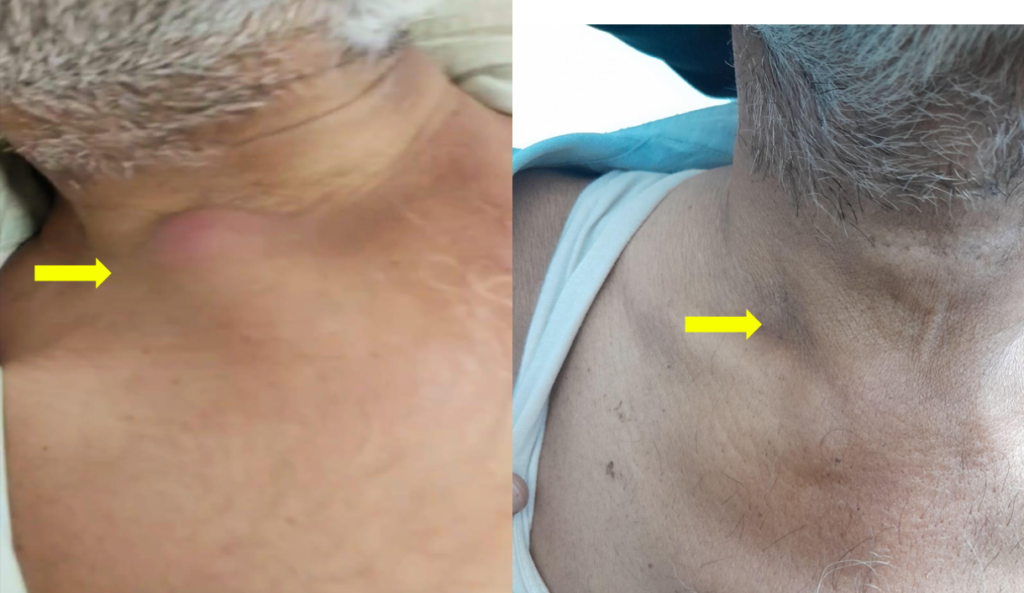
A man in his 50s, working as a farmer, with a history of poorly controlled type 2 diabetes mellitus (T2DM), high HbA1c, and a recently diagnosed hepatitis C virus (HCV) infection, presented to a tertiary care centre with persistent fever spikes accompanied by chills and rigours over the past month. He reported worsening right flank pain over the last 20 days, exacerbated by hip extension and occasionally radiating to the groin. Additionally, he described a gradually progressive painful swelling over the right upper chest for the past 10 days. The patient’s uncontrolled diabetes with high HbA1c and HCV infection contributed to rapid infection progression and complexity. Poor glycemic control causes impaired immune function, promoting bacterial growth and dissemination of E. coli outside the genitourinary tract. At the same time, the weakened immune response of the host due to uncontrolled HCV infection also leads to increased susceptibility to opportunistic infections, such as pyelonephritis and metastatic abscesses, resulting in a more severe clinical course. Upon physical examination, notable findings was a tender swelling over the right sternoclavicular joint, accompanied by localised warmth and erythema [Figure 1A].
Figure 1: Skin images of the patient showing an abscess over the right sternoclavicular joint with erythema [A, Pre treatment] and after content aspiration and treatment with antimicrobial [B].
[A] [B]
Examination of the right renal angle revealed tenderness as well. Other systemic examinations did not reveal any significant abnormalities.
The patient’s routine investigations revealed leukocytosis with neutrophilic predominance, prerenal-type acute kidney injury, and highly elevated inflammatory markers [Table 1]. Liver function tests were normal, but the patient was found to be reactive to HCV with a high viral load. His HbA1c was 16.6%, indicating poor glycemic control. Additional investigations ruled out TB and other infections, with negative results for urine acid-fast bacilli thrice, urine cartridge-based nucleic acid amplification test , and Mantoux test. Urine culture 3-4 days before admission grew E. coli. After admission, a routine urine examination showed plenty of pus cells. Repeat clean catch midstream urine culture and paired blood culture were sent, and both cultures grew E. coli with a similar antibiotic sensitivity susceptibility pattern [sensitive to piperacillin-tazobactam, co-trimoxazole, meropenem and amikacin].
|
Table 1: Basic and advanced investigations of the patient during hospitalization |
||||||||
|
Investigation |
SI unit |
Reference Range |
Day 1 |
Day 3 |
Day 5 |
Day 8 |
Day 12 |
Day 16 |
|
Hb |
g/dl |
13-17 |
9.3 |
9.2 |
9.1 |
8.9 |
9 |
9.1 |
|
MCV |
(fL) |
78-98 |
82 |
87 |
83 |
87 |
85 |
88 |
|
TLC (x1000) |
×103 cells/mm3 |
4-11 |
19.66K |
20.9K |
16.46K |
23.46K |
16.93K |
11.09k |
|
DLC (N/L/M) |
% |
40-70/20-40/2-8 |
84/7/6 |
72/13/14 |
82/12/13 |
79/12/7/0.5 |
74/16/6/1 |
70/20/08 |
|
Platelet |
×103 cells/mm3 |
150-400 |
301K |
353K |
470K |
535K |
603K |
410k |
|
Total Bilirubin |
mg/dl |
0.3 – 1.2 |
0.55 |
|
0.90 |
|
1 |
|
|
Direct Bilirubin |
mg/dl |
0 – 0.2 |
0.37 |
|
0.46 |
|
0.19 |
|
|
SGPT |
U/L |
0-50 |
34 |
|
40 |
|
37 |
|
|
SGOT |
U/L |
0-50 |
44 |
|
45 |
|
44 |
|
|
ALP |
U/L |
30-120 |
447 |
|
323 |
|
301 |
|
|
GGT |
U/L |
0-55 U/L |
173 |
|
160 |
|
155 |
|
|
Total protein |
g/dl |
6.6-8.3 gm/gl |
6.2 |
|
6.3 |
|
6.1 |
|
|
Albumin |
g/dl |
3.5-5.2 g/dl |
2.1 |
|
2.8 |
|
3.1 |
|
|
Globulin |
g/dl |
2.5-3.2g/dl |
4.1 |
|
3.5 |
|
3 |
|
|
Urea |
mg/dl |
17-43 |
112.5 |
78 |
|
22 |
|
|
|
Creatinine |
mg/dl |
0.72-1.18 |
1.51 |
1.10 |
|
0.45 |
|
|
|
PT/INR |
|
11.4/0.89 |
|
13/1.1 |
|
|
11/0.9 |
|
|
aPTT |
sec |
22 – 35 |
|
29 |
|
|
30 |
|
|
Reticulocyte count |
% |
0.8 – 1.8 |
1.98 |
|
|
|
|
|
|
Procalcitonin |
ng/ml |
< 0.5 |
|
0.38 |
|
|
|
|
|
LDH |
IU/L |
0 – 248 |
|
|
|
|
266 |
|
|
ESR |
mm/hr |
0-20 |
101 |
|
|
25 |
|
17 |
|
CRP |
mg/L |
0-1 |
230 |
|
|
101 |
|
61 |
|
Folate |
ng/ml |
> 5 |
|
|
|
|
|
|
|
HbA1c |
% |
4.5 – 5.5 |
|
16.6% |
|
|
|
|
Blood culture, Urine culture, and Pus culture – E.coli
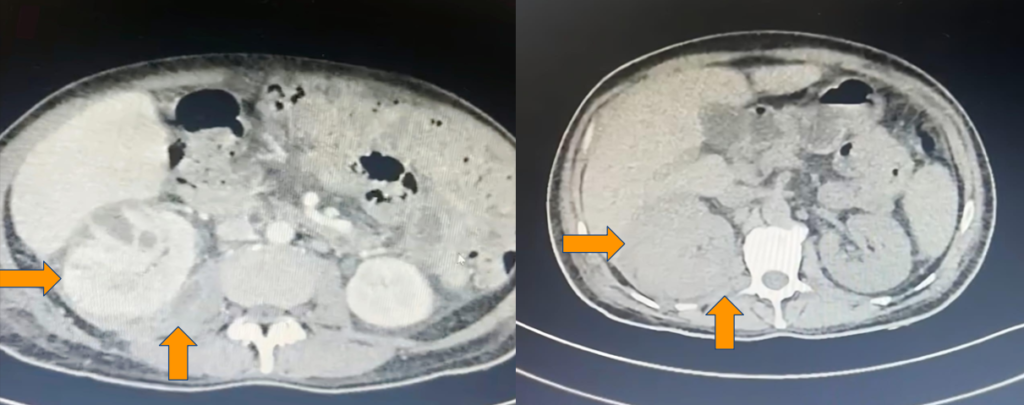
The patient was further investigated for a soft tissue abscess with septicemia. Contrast-enhanced computed tomography (CECT) scan of the thorax and abdomen suggested right upper chest collection, bilateral pyelonephritis (right > left), and right psoas abscess [Figure 2A]. Aspirated pus from the chest wall also grew E. coli with similar antibiotic sensitivity susceptibility pattern, confirming metastatic E. coli infection, with the probable source from UTI.
TB and Pott’s spine were ruled out due to the absence of night sweats, significant weight loss, close contact with a person having active TB infection, cough, cold abscess, and negative thorough workup for TB. Given the patient’s age, uncontrolled blood sugar levels, overcrowding, and poor hygienic practices at home, renal abscess was also considered and was ruled out by CECT abdomen.
A urine routine examination showed plenty of pus cells, and urine culture grew E. coli sensitive to meropenem. Initially, the patient was treated for a complicated UTI caused by E. coli with intravenous meropenem 1 gram thrice daily by infusion pump over 3 hours for 2 weeks. Injection Clindamycin 600 mg intravenous thrice daily, was later added for the chest wall abscess to cover anaerobic organisms. Strict glycemic control was ensured with bolus and basal insulin. Blood and pus cultures also yielded E. coli with the same antibiotic sensitivity pattern, so meropenem was continued, and clindamycin was stopped. Despite 2-weeks of meropenem administration, the patient continued to have a fever with daily spikes and persistent lower back pain. The patient’s risk factors raised concerns about MDR E. coli, particularly carbapenem-resistant strains, where mechanisms like carbapenemase production or efflux pumps can render carbapenems ineffective, necessitating careful antibiotic selection. Concern was raised about the inoculum effect, where a high bacterial load might reduce the effectiveness of antibiotics like meropenem. Given this, amikacin, an aminoglycoside with broad activity against gram-negative organisms, including carbapenem-resistant strains of E. coli, was added to the treatment regimen. This decision was based on empirical evidence of increasing resistance patterns in the region, where E. coli and other Gram-negative pathogens are becoming increasingly resistant to beta-lactams, including carbapenems. Amikacin was chosen for its potent activity against many Gram-negative bacteria, including those resistant to other antibiotics, and because it is often effective in severe infections like metastatic abscesses.
Figure 2: Abdominal contrast-enhanced computed tomography images of the patient revealed a right enlarged kidney with a psoas abscess [A, Before treatment], followed by recovery post-treatment [B].
[A] [B]
After the addition of amikacin, the patient’s clinical condition improved significantly. He became afebrile after three days and showed signs of resolution of the chest wall abscess [Figure 1B]. This improvement confirmed the utility of amikacin in managing this resistant infection.
The patient was discharged on Outpatient Parenteral Antimicrobial Therapy (OPAT) with amikacin for a total of 14 days. Follow-up contrast CT imaging showed complete resolution of the chest wall collection and psoas abscess, reducing kidney bulkiness compared to prior imaging [Figure 2B]. Routine investigations, including ESR and CRP, returned to normal, and the patient’s blood sugar levels were better controlled with antidiabetic medications at home.
DISCUSSION
Despite being a rare but documented complication, there is limited literature on the occurrence of psoas abscess and chest wall abscess secondary to disseminated UTIs. The patient, in this case, presented with several risk factors, including uncontrolled diabetes, advanced age, hepatitis C infection with a high viral load, and poor hygiene practices. These factors likely contributed to the initial UTI and its subsequent dissemination to distant sites by impairing immune system function.7
Iliopsoas abscesses are associated with a high mortality rate, reported to be as high as 19%.8 Typical symptoms include fever, flank pain, anorexia, weight loss, a palpable mass in the flank, and reduced hip mobility. Laboratory findings often show leukocytosis, elevated C-reactive protein, and a high erythrocyte sedimentation rate. The diagnosis of psoas abscess is frequently delayed due to non-specific presenting symptoms, with only 30% of patients displaying the characteristic triad of fever, flank pain, and reduced hip joint mobility.9 Staphylococcus aureus is the most common cause of primary psoas abscesses, whereas E. coli is more frequently associated with secondary cases. The anatomical proximity of the psoas muscle to major abdominal and pelvic organs facilitates the spread of infections from these regions, potentially extending into the posterior mediastinum or anterior thigh.
In our case, the metastatic spread of E. coli via the hematogenous route also resulted in a chest wall abscess. This is an infrequent presentation, as chest wall abscesses are more frequently associated with mycobacterial infections. The mainstay of treatment of such primary nontuberculous chest wall abscess includes antibiotic therapy and prompt drainage. In some cases, complex reconstructive surgeries may be required.10,11,12
The emergence of carbapenem-resistant E. coli strains poses a significant challenge in treating metastatic infections caused by them. Carbapenems are a class of antibiotics with a broad spectrum of activity, particularly against gram-negative pathogens producing chromosomal cephalosporinases and extended-spectrum beta-lactamases.13 However, the increasing prevalence of carbapenem-hydrolyzing beta-lactamases has severely limited the effectiveness of these drugs, highlighting the pressing need for new antimicrobial strategies and stewardship.14
Carbapenem resistance was noted in our patient, necessitating alternative antibiotics such as aminoglycosides. Aminoglycosides can be effective against carbapenem-resistant gram-negative bacteria with good tissue penetration and play a crucial role in managing such infections.15 It is essential to consider resistance patterns and risk factors such as uncontrolled diabetes, advanced age, and poor hygiene while selecting appropriate antibiotic therapy.
Metastatic infection can even occur in diabetic patients with an unknown source, as seen in cases leading to brain abscesses and septic lung emboli, underscoring the varied and severe nature of such infections.16
In diabetic patients, early detection of potential metastatic infection is critical. Clinicians should be vigilant for signs of worsening infection in a patient with UTI despite appropriate treatment, such as persistent fever spikes, localised pain in distant sites (e.g., flank pain, back pain, or chest pain), and new-onset painful and erythematous chest swellings. Studies suggest that high blood glucose levels can predict the likelihood of metastatic infections, as hyperglycemia impairs neutrophil function and enhances bacterial survival and dissemination to distant sites.
The rise of MDR E. coli infections is a growing concern in community and healthcare settings. Community-acquired MDR infections pose a significant public health threat due to their potential to spread rapidly and cause severe complications. The presence of MDR E. coli in the community can lead to limited treatment options, prolonged hospital stays, and increased healthcare costs. Public health initiatives must focus on preventing the spread of these infections through improved hygiene practices, judicious use of antibiotics, and increased awareness among healthcare providers and patients.17
CONCLUSION
The psoas and chest wall abscesses as sequelae of disseminated UTIs may occur in patients with advanced age and poorly controlled diabetes mellitus. The escalating prevalence of carbapenem-resistant Enterobacterales, notably E.coli, is present in the community, and antibiotic stewardship practices are required to curtail them. The nonspecific clinical manifestations associated with psoas and chest wall abscesses result in delayed diagnoses. Comorbidities, such as uncontrolled diabetes and hepatitis C, may facilitate the dissemination of UTIs and complicate therapeutic approaches.
INFORMED CONSENT
Written informed consents were obtained from the patient. The confidentiality of the patients was maintained in the article.
CONFLICTS OF INTEREST STATEMENT
The authors declare no conflict of interest.
SOURCE OF FUNDING
None
AUTHOR’S CONTRIBUTION
AK: Data collection; Analysis; Writing the draft
MA: Conceptualization; Investigation; Methodology; Resources; Review & Editing
PKP: Conceptualization; Investigation; Methodology; Resources; Review & Editing
REFERENCES
Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113 Suppl 1A:5S-13S.
Salehi M, Jafari S, Mirzashahi B, et al. Escherichia coli and spondylodiscitis: A review of the literature. Jundishapur J Microbio. 2019;12(12):e99694.
Sakran W, Bisharat N. Primary chest wall abscess caused by Escherichia coli costochondritis. Am J Med Sci. 2011;342(3):241-6.
Poirel L, Madec JY, Lupo A, et al. Antimicrobial Resistance in Escherichia coli. Microbiol Spectr. 2018;6(4):10.1128/microbiolspec.arba-0026-2017.
Castanheira M, Davis AP, Mendes RE, Serio AW, Krause KM, Flamm RK. In Vitro Activity of Plazomicin against Gram-Negative and Gram-Positive Isolates Collected from U.S. Hospitals and Comparative Activities of Aminoglycosides against Carbapenem-Resistant Enterobacteriaceae and Isolates Carrying Carbapenemase Genes. Antimicrob Agents Chemother. 2018;62(8):e00313-18.
Tamma PD, Heil EL, Justo JA, Mathers AJ, Satlin MJ, Bonomo RA. Infectious Diseases Society of America 2024 Guidance on the Treatment of Antimicrobial-Resistant Gram-Negative Infections. Clin Infect Dis. Published online August 7, 2024. doi:10.1093/cid/ciae403
Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;26(2):510-3.
Audia S, Martha B, Grappin M, et al. Les abcès pyogènes secondaires du psoas: à propos de six cas et revue de la littérature [Pyogenic psoas abscess: six cases and review of the literature]. Rev Med Interne. 2006;27(11):828-35.
Sato M, Iwasa Y, Otsubo S, et al. Psoas abscess in hemodialysis patients. Int Urol Nephrol. 2010;42(4):1113-6.
Y, Yamamura J, Masuda N, et al.: Primary chest wall abscess mimicking a breast tumor that occurred after blunt chest trauma: a case report. Case Rep Med. 2014, 620876. 10.1155/2014/620876
Grondin SC, Gelfand GJ: Surgical treatment of chest wall infections. Adult Chest Surgery. Sugarbaker DJ (ed): McGraw-Hill, New York, NY; 2014.
Bouaziz MC, Jelassi H, Chaabane S, Ladeb MF, Miled-Mrad KB: Imaging of chest wall infections. Skeletal Radiol. 2009, 38:1127-35. 10.1007/s00256-008-0636-z
Tamma PD, Rodriguez-Bano J. The Use of Noncarbapenem β-Lactams for the Treatment of Extended-Spectrum β-Lactamase Infections. Clin Infect Dis. 2017;64(7):972-80.
van Duin D, Doi Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence. 2017;8(4):460-9.
Poole K. Aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2005;49(2):479-87.
Routhu B, Kumar S, Panda PK, Kant R. Metastatic Infection of Unknown Origin in a Diabetic and Alcoholic. JASPI. 2024;2(1):25-8.
Logan LK, Weinstein RA. The Epidemiology of Carbapenem-Resistant Enterobacteriaceae: The Impact and Evolution of a Global Menace. J Infect Dis. 2017;215(suppl_1):S28-S36.
Submit a Manuscript:
Copyright © Author(s) 2024. JASPI- Journal of Antimicrobial Stewardship Practices and Infectious Diseases.

