Aspergillus oryzae Isolated from Lung Tissue Sample in a Chronic Respiratory Illness Patient from South India
JASPI June 2024/ Volume 2/Issue 2
Copyright: © Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
April-June 30, 2024
Muthiah V, Gopal V, Murugesan M. Aspergillus oryzae Isolated from Lung Tissue Sample in a Chronic Respiratory Illness Patient from South India. JASPI. 2024;2(2)46-50 DOI: 10.62541/jaspi030
ABSTRACT
Opportunistic fungal infection by Aspergillus oryzae in patients with chronic respiratory condition is rare in India. A 52-year-old male with a history of diabetes mellitus, chronic smoking, and alcoholism presented with chronic cough, breathlessness, and recurrent blood in sputum. CT imaging revealed features consistent with old pulmonary tuberculosis sequelae and the presence of a right lung lower lobe aspergilloma. The patient underwent a right lower lobectomy, and histopathological examination confirmed severe acute and chronic bronchiolitis with bronchiectasis and organizing pneumonia, indicative of fungal ball formation. Microbiological analysis identified Aspergillus species, possibly A. flavus complex, with sequencing confirming A. oryzae. This case is unique as A. oryzae is typically associated with food fermentation and rarely implicated in invasive lung infections, especially outside regions of traditional exposure, like Japan. The rarity of this presentation underscores the importance of considering unusual pathogens in clinical practice and utilizing advanced diagnostic techniques such as sequencing for accurate identification. The successful outcome of this case, with prolonged antifungal therapy based on microbiological confirmation and clinical follow-up, suggests the importance of personalized treatment strategies. This case report adds to the growing body of evidence on the clinical significance and pathogenic potential of A. oryzae. It underscores the importance of continued vigilance and research in fungal infections.
KEYWORDS: Aspergilloma, Diabetes mellitus; Koji mold; Opportunistic fungal infections; Sequencing, smoker
INTRODUCTION
Most of the studies conducted on the speciation of Aspergillus causing CPA have shown that A. fumigatus is the most common cause, followed by A. niger, A. flavus, A. nidulans, A. terreus and other rare species.3 A. oryzae is a fungus widely used in traditional fermentation industries, including soy sauce, sake, bean curd seasoning and vinegar production.4 It is infrequent to isolate A.oryzae as it does not commonly appear as a human pathogen. Based on the review of the literature through the PubMed search engine, it was noticed that only very few case reports have been published in the past. A.oryzae has been documented as a causative pathogen in meningitis in a case of paranasal sinusitis and a patient with pulmonary aspergilloma.5-7 As this fungal infection in humans is extremely rare, cautious reporting should be done with adequate evidence and appropriate microbiological investigations, preferably with sequencing reports. A close taxonomical association is seen with A. flavus and A. oryzae with genome similarity. Hence, morphological findings and sequencing reports confirm the species’ pathogenicity in humans.
This report presents a rare case of pulmonary aspergilloma caused by A. oryzae, which was identified and confirmed using the sequencing technique.
CASE REPORT
A 52-year-old male patient, chronic smoker and alcoholic, who had a known case of diabetes mellitus presented to the outpatient clinic in mid of 2023 with complaints of cough with expectorations on and off for the past five years, which aggravated over the last six months, breathlessness and recurrent episodes of blood in sputum since two months. The patient was diagnosed with laboratory-confirmed pulmonary tuberculosis (PT) in 2015 and completed anti-tubercular therapy.
Owing to the history of tuberculosis, the patient’s sputum sample was sent for acid-fast bacilli (AFB) staining, which showed a negative finding. A sputum specimen was sent for Xpert MTB-RIF assay (Cepheid), which also showed negative results. In contrast, the computed tomography (CT) of the chest showed features suggestive of old PT, right lung lower lobe aspergilloma, and alveolar opacification in the upper and lower lobes of both lungs. A clinical diagnosis of old PT sequelae with suspicion of active fungal infection was made, and the patient was advised for the right lower lobectomy.
He underwent the procedure, and the postoperative period was uneventful. During the procedure, the right lower lobe mass was identified with a size of 5X5 cm2 in the apical portion of the lower lobe. Hence, the right lower lobe was excised along the mass in toto. The excised tissue was sent for histopathological examination (HPE) and bacterial and fungal culture and susceptibility testing. The patient was treated with IV antibiotics – levofloxacin 750 mg once daily and oral itraconazole 200 mg twice daily for two weeks and followed up after 14 days with the culture and HPE report.
Figure 1: Histopathological imaging of the lung tissue biopsy sample. (A) Microscopic sections studied from the lesion showed severe acute and chronic bronchiolitis with patchy fibrosis, consistent with bronchiectasis and organizing pneumonia; (B) Direct potassium hydroxide (KOH) mount of the tissue samples revealed few branching septate hyphae, suggestive of aspergillosis.
[A] [B]
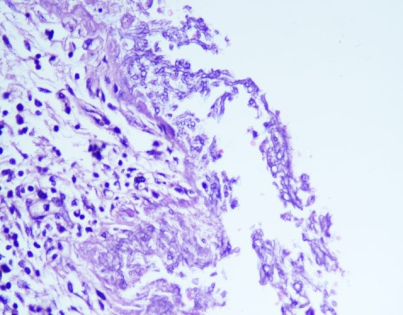
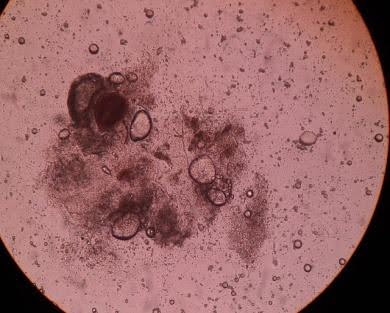

Microscopic sections studied from the lesion showed severe acute and chronic bronchiolitis with patchy fibrosis, consistent with bronchiectasis and organizing pneumonia [Fig 1A]. Special staining of the sections revealed the presence of fungal organisms showing slender hyphae with acute angle branching, suggestive of aspergillosis [Fig 1B].
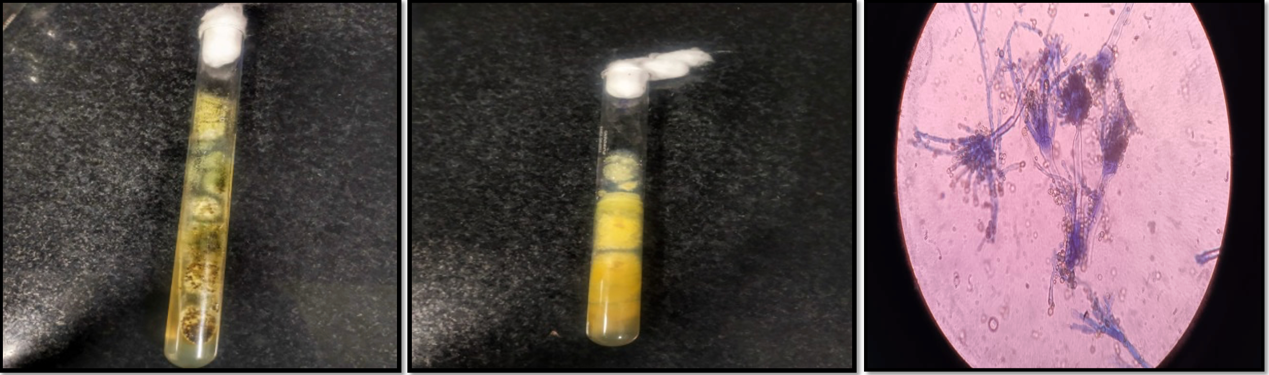
Aerobic bacterial culture and subculture from thioglycollate broth on MacConkey, blood agar and mannitol salt agar showed no growth. On Sabouraud’s Dextrose Agar, colonies appeared on the second day that looked granular and flat, and the colony was initially white, then turned to yellowish green on the fourth day and then to greenish brown on subsequent days [Figure 2]. Microscopically, it revealed conidial heads that are globose or radiate with loose columns. The conidia were large, approximately 5-8 μm in diameter, typically radiate, later splitting to form loose columns and were globose, pale green and had biseriate arrangements, but some heads with phialides borne directly on the vesicle. The organism was identified as an Aspergillus species, possibly a flavus complex. Since the color of the colony turned slightly brown on SDA media, which may be seen in older cultures, and phialides were longer than usual A. flavus on Lactophenol Cotton Blue (LPCB) mount, we sent the isolate for sequencing to confirm whether it is A. flavus or some other Aspergillus spp.
Figure 2: Mycological culture in Sabouraud’s Dextrose Agar and Microscopic features of the colonies. Colonies appeared on the second day, looking granular and flat. The colony was initially white and then turned yellowish-green on subsequent days. Microscopically, it revealed conidial heads that radiate with loose columns and were globose, pale green and had biseriate arrangements, but some heads with phialides borne directly on the vesicle.
Using universal primers, a polymerase chain reaction was performed per standard protocol to amplify the Internal transcribed spacer (ITS) gene. Universal ITS gene primers were selected from previous studies for complete amplification.8 The amplified products were checked on 1.5% agarose gel electrophoresis, and the molecular weight was checked using a molecular weight marker (100bp ladder) [Figure 3]. The sequencing reaction was done in a PCR thermal cycler (GeneAmp PCR System 9700, Applied Biosystems) using the BigDye Terminator v3.1 Cycle sequencing Kit (Applied Biosystems, USA). The sequence quality was checked using Sequence Scanner Software v1 (Applied Biosystems). Sequence alignment and required editing of the obtained sequences were carried out using MEGA 7.
Figure 3: PCR Gel electrophoresis. The image showed the amplified product that was checked on 1.5% agarose
gel electrophoresis.
The similarity index built in the NCBI’s BLAST program confirmed the amplified sequences belonging to the ITS gene. Based on the higher percentage similarity against the reference species, the species being investigated was assigned to Aspergillus oryzae NRRL 447.
Based on the histopathological and microbiological findings, oral itraconazole 200 mg twice daily was continued for 12 months. The patient is currently on follow-up as an outpatient with no complaints or complications.
DISCUSSION
We present a rare case of Aspergillus oryzae causing invasive fungal infection in the lungs, which presented as chronic invasive aspergillosis in a PT patient as sequelae. A.oryzae, called Koji Mold, has been extensively used in Japan to produce sake, miso, and soy sauce for over 1000 years.9 Very few case reports of A.oryzae are reported, mainly among those who worked in rice fields in Japan and those who are immunocompromised [Table 1]. In this case, we have noted that the patient belonged to South India and had no history of travelling to regions where this fungal entity is reported, which is a rarity in this region. Clinical diagnosis of aspergilloma helped in the early initiation of azoles. In laboratory diagnosis, the morphological findings from the lung tissue did not correlate with the common Aspergillus spp. It was doubtful that the microbiologist would confirm it with sequencing. Most centres do not have sequencing facilities or do not confirm the doubtful isolates with sequencing, especially in mycological samples, leading to underreporting of uncommon pathogens. This case report highlights the importance of sequencing uncommon isolates to confirm the pathogenicity of novel fungal pathogens and the probability of pathogenesis in existing filamentous saprophytic fungi like A. oryzae.
Table 1: Review of case reports on Aspergillus oryzae causing pulmonary infections.
|
Author |
Year |
Location |
Clinical History |
Treatment |
|
Gordon et al.,5 |
1976 |
New York |
A. oryzae meningitis |
Amphotericin B and flucytosine. |
|
Schwetz et al.,12 |
2007 |
Austria |
A case of peritonitis caused by A. oryzae in a man on CAPD therapy |
Amphotericin B and caspofungin, followed by itraconazole |
|
Wilson et al.,13 |
2018 |
California |
Chronic meningitis due to A. oryzae |
Not given |
|
Kume et al.,14 |
2022 |
Japan |
Bronchial asthma due to A. oryzae |
Mainly anti-allergics and bronchodilators |
The CPAnet consensus statement published in 2018 stated that chronic pulmonary aspergillosis is a neglected fungal infection, and many fundamental questions need more collaborative research activities to address them.10 Species identification is based on morphology, which is nowadays considered to discriminate the Aspergillus species insufficiently.10 Accurate species-level identification of Aspergillus and maintaining a fungal registry is crucial as we look at azole-resistant fungi. In addition, antifungal testing criteria are not available for uncommon species, which is clinically challenging to correlate if the patient does not respond to the commonly used azoles. Hence, doubtful morphology requires confirmation with sequencing. IDSA recommends that antifungal therapy should be continued for 6-12 weeks.11 However, prolonged therapy based on microbiological confirmation and clinical follow-up helped achieve a good prognosis in the above patient.
Furthermore, the identification of Aspergillus oryzae as a causative agent prompts a reevaluation of its clinical significance and pathogenic potential. This case emphasizes the need for continued surveillance and research into less conventional fungal pathogens, expanding our understanding of their ecological niches and potential impact on human health. The successful application of sequencing methodologies facilitates a precise diagnosis and enables a targeted and timely therapeutic intervention.
CONCLUSION
Ultimately, this case report contributes to the growing body of literature on fungal infections, urging clinicians and researchers to remain vigilant in their diagnostic approaches and treatment strategies, particularly as new insights into fungal diversity and pathogenicity continue to emerge through advanced molecular techniques. In addition, prompt diagnosis and appropriate antifungal therapy duration must be critically followed to ensure microbiological and clinical cure.
ACKNOWLEDGEMENT
The authors thank and acknowledge the Department of Cardiothoracic surgery and OT support staff of MMHRC for their clinical support in management of the case.
INFORMED CONSENT
Written informed consent was obtained from the patient. Confidentiality of the patient was maintained in the article.
CONFLICTS OF INTEREST STATEMENT
The authors declare no conflict of interest.
SOURCE OF FUNDING
None
AUTHOR’S CONTRIBUTION
VM: Conceptualization, Microbiological analysis; Writing the draft
VG: Clinical Resources; Writing the draft
MM: Supervision; Validation; Review and editing
REFERENCES
1. Mahajan M, Prasanna S, Das NK, Mahajan N. Case series of invasive lung infections by Aspergillus species and zygomycosis among post-COVID-19 and post-transplant individuals. J Family Med Prim Care. 2022;11(11):7469-75.
2. Ullmann AJ, Aguado JM, Arikan-Akdagli S, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24 Suppl 1:e1-e38.
3. Godet C, Laurent F, Bergeron A, et al. CT Imaging Assessment of Response to Treatment in Chronic Pulmonary Aspergillosis. Chest. 2016;150(1):139-47.
4. Machida M, Asai K, Sano M, et al. Genome sequencing and analysis of Aspergillus oryzae. Nature. 2005;438(7071):1157-61.
4. Gordon MA, Holzman RS, Senter H, Lapa EW, Kupersmith MJ. Aspergillus oryzae meningitis. JAMA. 1976;235(19):2122-3.
5. Byard RW, Bonin RA, Haq AU. Invasion of paranasal sinuses by Aspergillus oryzae. Mycopathologia. 1986;96(1):41-43.
6. Liao WQ, Shao JZ, Li SQ, et al. Mycological identification of pulmonary aspergilloma caused by Aspergillus oryzae with proliferating heads. Chin Med J (Engl). 1988;101(8):601-4.
7. Martin KJ, Rygiewicz PT. Fungal-specific PCR primers developed for analysis of the ITS region of environmental DNA extracts. BMC Microbiol. 2005;5:28.
8. Kitamoto N, Go M, Shibayama T, et al. Molecular cloning, purification and characterization of two endo-1,4-beta-glucanases from Aspergillus oryzae KBN616. Appl Microbiol Biotechnol. 1996;46(5-6):538-44.
9. Chawla K, Kosaraju K, Rayasam S, Mukhopadhyay C. Clinico-microbiological profile of chronic pulmonary aspergillosis from a tertiary care centre in southern India. J Clin Diagn Res. 2013;7(12):2712-5.
10. Patterson TF, Thompson GR, Denning DW, et al. Clinical Practice Guideline for the Diagnosis and Management of Aspergillosis: 2016 Update by IDSA. Clinical Infectious Diseases. 2016;63(4):e1-e60.
11. Schwetz I, Horina J, Buzina W, Roob J, Olschewski H, Krause R. Aspergillus oryzae peritonitis in CAPD: case report and review of the literature. Am J Kidney Dis. 2007;49(5):701-4.
12. Wilson MR, O’Donovan BD, Gelfand JM, et al. Chronic Meningitis Investigated via Metagenomic Next-Generation Sequencing. JAMA Neurol. 2018;75(8):947-55.
13. Kume H, Tomita H, Fukuhara A. [A CASE OF BRONCHIAL ASTHMA CAUSED BY EXPOSURE TO ASPERGILLUS ORYZAE IN A JAPANESE RICE WINE BREWERY WORKER]. Arerugi. 2022;71(4):321-32.
Submit a Manuscript:
Copyright © Author(s) 2024. JASPI- Journal of Antimicrobial Stewardship Practices and Infectious Diseases.

