Plasmodium Falciparum associated Rhabdomyolysis
JASPI March 2024/ Volume 2/Issue 1
Copyright: © Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Badole PG, Pandit VR, Singh J.Plasmodium Falciparum Associated Rhabdomyolysis. JASPI.2024;2(1):20-24 DOI: 10.62541/jaspi019
ABSTRACT
Rhabdomyolysis-induced acute kidney injury (AKI) is highly unusual in P. falciparum-infected patients. Only a handful of cases have been described in the form of case reports, and the clear pathophysiology behind malaria producing the rhabdomyolysis is unclear. A 55-year-old man with a past medical history of 12 years of alcohol abuse presented to the Emergency Department with a 5-day history of on and off fevers and a 1-day history of pain in the abdomen, vomiting, and shortness of breath. Following initial investigations, the patient was found positive for Plasmodium falciparum infection. The patient was treated with intravenous (IV) artesunate, and on day-2 of admission, the patient developed anuria, severe respiratory distress, and bradycardia and had to be intubated and upgraded to intensive level care; further evaluation revealed elevated serum creatinine and myoglobinuria and diagnosis of secondary rhabdomyolysis and AKI. He was managed with forced alkaline diuresis, and a urine output higher than 200ml/hour was maintained with IV fluids. The patient consequently also developed symptoms of alcohol withdrawal, and lorazepam was added for management. On days 5-6 of admission, his symptoms started to improve, and his serum markers of rhabdomyolysis and AKI normalized. The patient was extubated, discharged, remains in follow-up, and has no residual sequelae due to this episode of illness.
KEYWORDS: Complicated malaria, secondary AKI, intravenous hydration, hemodialysis, myoglobinuria
INTRODUCTION
Five Plasmodium species can infect humans: P. falciparum, P. ovale, P. vivax, P. malariae, and P. knowlesi.1 The disease has a high prevalence in Southeast Asia and Sub-Saharan Africa.2 India is the world’s biggest democracy, the second most populous country with over 1.2 billion people, and the seventh largest by area. The percentage of P. falciparum infections has gradually increased from 39% in 1995 to 63.84% in 2020. The mortality and morbidity due to the disease remain high in endemic regions where the protozoan is constantly transmitted and has an increasing rate of resistance to anti-malarial agents.
P. falciparum is the cause of one of the most aggressive forms of malarial illness, which can involve multiple organ systems. Acute kidney injury (AKI) is one of the known complications of P. falciparum infection. However, the usual mechanism behind this is hypovolemia, inflammatory cytokine release, direct tubular toxicity, and obstruction of renal microvasculature. Rhabdomyolysis is an uncommon cause of AKI in malaria and is usually caused by traumatic crush injuries or direct insults to the musculature. The classical manifestations observed are myalgia, weakness, and tea-colored urine.3 However, less than 10% of patients show this classic triad, and more than 50% do not have muscle pain or weakness.4 Skeletal muscle damage is common in malaria on biochemical examination, like increased myoglobin levels, but the development of overt myonecrosis in these patients is rare. We want to report this fascinating, unusual case with Plasmodium falciparum associated with rhabdomyolysis.
CASE REPORT
A 55-year-old man resident of Chhattisgarh, India, presented with a five-day history of intermittent fever and a one-day history of abdominal pain, vomiting, and difficulty in breathing. The patient had a 12-year history of alcohol abuse, with the last alcohol intake being seven days before admission. He had no history of statin use or any other medications, heavy exertion, or seizures. He was admitted, and a rapid immunochromatographic test was positive for P. falciparum, confirmed on peripheral smear examination with a parasitic index of 2%. The patient was started on IV artesunate at the dose of 2.4 mg/kg body weight, along with an injection of doxycycline. His thyroid profile was normal. Tests for Dengue IgM, Scrub Typhus IgM, and leptospirosis were negative. Blood culture for aerobic bacteria showed no growth. His calcium and phosphorous levels were within normal limits on the first report.
There was no evidence of muscle abscess or deep vein thrombosis (DVT), as evidenced by ultrasound of the lower limb. On the second day of admission, the patient became afebrile but developed irritability and delirium followed by tachypnea. He was sweating profusely with a heart rate of 130 per minute. An electrocardiogram showed sinus tachycardia. He then developed bradycardia, and his oxygen saturation began to drop, for which he was shifted to the medical intensive care unit (ICU) for further management. He was intubated, and mechanical ventilation was started to achieve adequate oxygen saturation. He was also started on lorazepam for the possibility of alcohol withdrawal. On the same day, we noticed high-colored urine and decreased urine output progressing to oliguria. On further investigation, the patient’s creatinine phosphokinase (CPK) levels were high, 9978 U/L, lactate dehydrogenase was 2,125 U/L, and myoglobin was present in the urine. We started him on aggressive intravenous hydration with normal saline and 5% dextrose, along with intravenous sodium bicarbonate for urine alkalinization. We maintained a urine output of approximately 200 ml per hour. The trend of his laboratory investigations on subsequent days is summarized in Table 1. Artesunate was continued for seven days, including three days of artemisinin combined therapy.
Table 1- Trends of patient’s laboratory values during the first five days of hospital admission
|
Parameters (in units) |
Normal values |
Days of inpatient care |
|||||
|
Day 0 (at admission) |
Day 1 |
Day 2 |
Day 3 |
Day 4 |
Day 5 |
||
|
Hb (in g/dL) |
12-16 |
12.6 |
11.9 |
11.5 |
10.1 |
9.0 |
7.7 |
|
TLC in cells/µL) |
4000-11000 |
5110 |
5400 |
9000 |
11390 |
10700 |
12560 |
|
Platelet count (in cells/µL) |
150000-450000 |
22000 |
24000 |
18000 |
25000 |
87000 |
132000 |
|
Blood Urea (in mg/dL) |
15-39 |
29 |
56 |
128 |
129 |
83 |
77 |
|
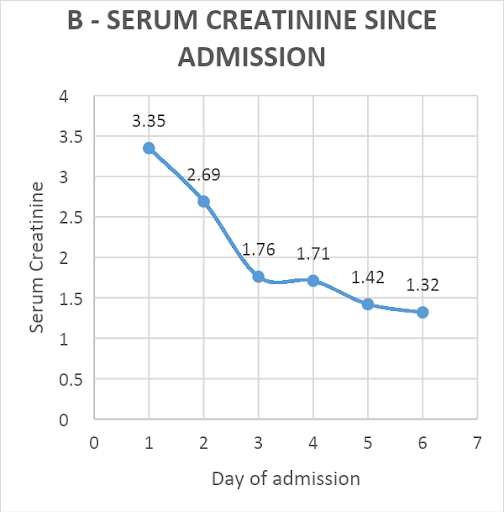
Serum creatinine (in mg/dL) |
0.9-1.3 |
1.29 |
3.35 |
2.69 |
1.76 |
1.71 |
1.42 |
|
Total bilirubin (in mg/dL) |
0.3-1.3 |
9.89 |
13.54 |
7.46 |
5.82 |
4.54 |
3.8 |
|
Direct Bilirubin (in mg/dL) |
0.1-0.3 |
5.26 |
7.96 |
4.17 |
3.28 |
2.3 |
1.8 |
|
AST (in U/L) |
12-38 |
49 |
74 |
282 |
192 |
110 |
114 |
|
ALT (in U/L) |
7-41 |
66 |
44 |
91 |
96 |
89 |
84 |
|
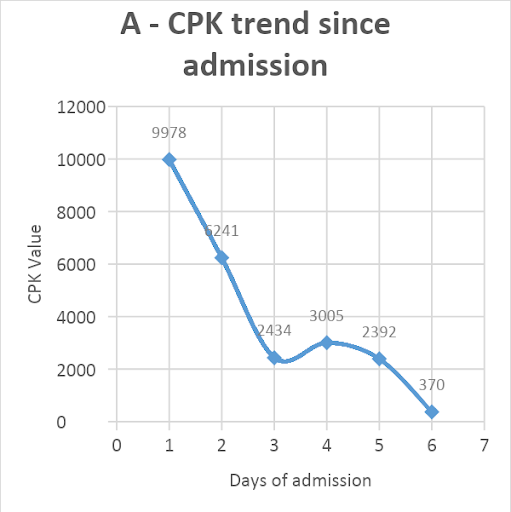
CPK (in U/L) |
9978 |
6241 |
2434 |
3005 |
2392 |
||
|
LDH (in U/L) |
2125 |
1643 |
1328 |
1014 |
901 |
||
(Hb- Hemoglobin, TLC- Total leukocyte count, PLT- Platelet count, AST- Aspartate transaminase, ALT-Alanine transaminase, CPK- Creatinine phosphokinase, LDH- Lactate dehydrogenase)
Hemodialysis was not done as the creatinine level improved, and adequate urine output was achieved with the given treatment. The patient responded well to the treatment and started regaining consciousness. He had a full Glasgow coma score by day-4 of admission. One unit of packed red blood cells was transfused for a rapid fall in hemoglobin from 12.6 to 7.7 gm%. His CPK levels decreased from 9978 to 370 U/L [Figure 1A]. The creatinine level improved from 3.35mg/dl to 1.32 mg/dl over five days [Figure 1B]. The patient was extubated on day-5. Thick blood smear became negative on repeat examination after day-5 of starting artesunate. He was eventually discharged on day-9 of admission, remains in follow-up, and has no residual sequelae from the disease.
Figure 1: Trend of patient’s serum creatinine phosphokinase (CPK, U/L) values (A) and creatinine (U/L) values (B) since admission
DISCUSSION
Rhabdomyolysis is a breakdown of skeletal muscle and leakage of its intracellular contents into the bloodstream. Intracellular contents such as myoglobin, sarcoplasmic proteins (lactate dehydrogenase, creatinine phosphokinase, aldolase, alanine, and aspartate aminotransferase) and electrolytes (primarily potassium and phosphate) come into the plasma and their elevated levels are the primary cause of the pathology associated with rhabdomyolysis.4 The common causes of rhabdomyolysis are trauma, exertion, muscle hypoxia, infections, metabolic and electrolyte disorders, drugs, toxins, and genetic defects.5 The causes can be split into traumatic and non-traumatic insults. Causes are listed in Table 2.5,6
Table 2 – Causes of rhabdomyolysis
|
Traumatic Rhabdomyolysis |
Non-traumatic Rhabdomyolysis |
|
Accidents Earthquakes Prolonged somnolescent state Compartment syndrome Muscle overuse |
Drugs and Toxins Alcohol Colchicine Carbon monoxide Statins Daptomycin Cocaine Insect and snake bites Infections Pyomyositis (S. aureus) Septic shock Toxic shock syndrome Dyselectrolytemia Hypokalemia Hypophosphatemia Hypocalcemia Hypercalcemia Severe dehydration Congenital and acquired myopathies Disorders of glucose metabolism Disorders of lipid metabolism Brody’s myopathy Autoimmune myositis Others Neurolept malignant syndrome Malignant hyperthermia Hyperosmolar conditions |
In the present case, the patient was suffering from P falciparum malaria and developed AKI secondary to rhabdomyolysis, which is an unusual presentation in malaria. AKI in falciparum malaria is usually secondary to microvascular obstruction, endothelial activation, and cytokine-mediated acute tubular necrosis and kidney injury.7 This current iteration of AKI seen in this case secondary to rhabdomyolysis in falciparum malaria infection is atypical and has only been described in a handful of case reports.8-12 The pathogenesis of skeletal muscle damage in falciparum malaria is unclear. It has been found that the markers of skeletal muscle damage progress as the anti-malarial treatment is started, and hence, a hypothesis has been put forward stating that the possible cause is the exacerbation of the inflammatory response associated with parasite killing by the drug. This occurs in skeletal muscle due to increased sequestration of P. falciparum in the skeletal muscles.13
Management of rhabdomyolysis-induced AKI is to ensure sufficient fluid hydration to maintain high urine output. IV fluids must be titrated to maintain a urine output of 200 to 300 mL/hour, and serial CPK levels must be monitored daily to document downtrend levels. As mentioned earlier, CPK levels of more than 5,000 IU/L have increased the risk of the development of AKI. In patients with CPK levels, less than 5,000 IU/L extensive volume fluid resuscitation is discouraged as they are less likely to develop AKI. Forceful alkaline diuresis can be considered in severe cases where the CPK is more than 30,000 IU/L.4 In case of unresponsiveness of AKI to fluid resuscitation, hemodialysis may be considered; however, limited benefit has been documented in the literature.14
A positive history of alcohol abuse further complicated this case, and the patient developed symptoms of alcohol withdrawal; however, the patient never experienced any episodes of seizures, and the alcohol withdrawal was promptly managed. Alcohol use also contributes to the development of rhabdomyolysis via two different mechanisms – immobilization and coma are causative factors in short-term alcohol intoxication versus disturbances in electrolyte and acid-base balance in long-term alcohol abuse. It is believed that the direct toxic effect of alcohol on muscles, via disruption of adenosine triphosphate pump function, muscle membrane, or cytochrome P450 induction, leads to the disintegration of skeletal muscle.15
CONCLUSION
This case highlights an unusual presentation of falciparum malaria in the form of rhabdomyolysis. This may occur due to the organism per se or an immune-mediated inflammatory reaction after its death following the initiation of anti-malarial therapy. Such an occurrence needs to be identified at the earliest in cases of severe malaria with a high parasite index as it is an amenable but life-threatening complication. An early aggressive hydration therapy can be started to prevent severe kidney injury.
INFORMED CONSENT
Written informed consent was obtained from the patient. Confidentiality of the patient was maintained in the article.
CONFLICT OF INTERESTS STATEMENT
The authors declare no conflict of interest.
SOURCE OF FUNDING
None
AUTHORS’ CONTRIBUTIONS
PGB: Conceptualization; Review & editing the final manuscript
VRP: Supervision, Validation
JV: Writing the draft
REFERENCES
-
Siagian FE, Ronny R, Sirra AI, Susiantoro U, Siregar M. Malaria related myalgia-arthralgia: an imported case report treated with anti-malarial drug. International Journal of Basic & Clinical Pharmacology. 2020;9(10):1603-6.
-
Patouillard E, Griffin J, Bhatt S, Ghani A, Cibulskis R. Global investment targets for malaria control and elimination between 2016 and 2030. BMJ Glob Health. 2017;2(2):e000176.
-
Bagley WH, Yang H, Shah KH. Rhabdomyolysis. Intern Emerg Med. 2007;2(3):210-218.
-
Torres PA, Helmstetter JA, Kaye AM, Kaye AD. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15(1):58-69.
-
Stanley M, Chippa V, Aeddula NR, Quintanilla Rodriguez BS, Adigun R. Rhabdomyolysis. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
-
Aleckovic-Halilovic M, Pjanic M, Mesic E, Storrar J, Woywodt A. From quail to earthquakes and human conflict: a historical perspective of rhabdomyolysis. Clin Kidney J. 2020;14(4):1088-96.
-
Plewes K, Turner GD, Dondorp AM. Pathophysiology, clinical presentation, and treatment of coma and acute kidney injury complicating falciparum malaria. Current opinion in infectious diseases. 2018;31(1):69.
-
Yong KP, Tan BH, Low CY. Severe falciparum malaria with dengue coinfection complicated by rhabdomyolysis and acute kidney injury: an unusual case with myoglobinemia, myoglobinuria but normal serum creatine kinase. BMC Infect Dis. 2012;12:364.
-
Reynaud F, Mallet L, Lyon A, Rodolfo JM. Rhabdomyolysis and acute renal failure in Plasmodium falciparum malaria. Nephrol Dial Transplant. 2005;20(4):847.
-
Mishra SK, Pati SS, Mahanta KC, Mohanty S. Rhabdomyolysis in falciparum malaria–a series of twelve cases (five children and seven adults). Trop Doct. 2010;40(2):87-88.
-
Sinniah R, Lye W. Acute renal failure from myoglobinuria secondary to myositis from severe falciparum malaria. Am J Nephrol. 2000;20(4):339-43.
-
Allo JC, Vincent F, Barboteu M, Schlemmer B. Falciparum malaria: an infectious cause of rhabdomyolysis and acute renal failure. Nephrol Dial Transplant. 1997;12(9):2033-4.
-
Davis TM, Supanaranond W, Pukrittayakamee S, Holloway P, Chubb P, White NJ. Progression of skeletal muscle damage during treatment of severe falciparum malaria. Acta Trop. 2000;76(3):271-6.
-
Holt S, Moore K. Pathogenesis of renal failure in rhabdomyolysis: the role of myoglobin. Exp Nephrol. 2000;8(2):72-6.
-
Riggs JE. Alcohol-associated rhabdomyolysis: ethanol induction of cytochrome P450 may potentiate myotoxicity. Clin Neuropharmacol. 1998;21(6):363-4.
Submit a Manuscript:
Copyright © Author(s) 2024. JASPI- Journal of Antimicrobial Stewardship Practices and Infectious Diseases.

