Disseminated Mucormycosis Masquerading as Invasive Pulmonary Aspergillosis
JASPI June 2024/ Volume 2/Issue 2
Copyright: © Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
April-June 30, 2024
Vamadevan S, Phulware RH, Panda PK, Panwar VK.Disseminated Mucormycosis Masquerading as Invasive Pulmonary Aspergillosis. JASPI. 2024;2(2)51–56 DOI: 10.62541/jaspi031
ABSTRACT
Pulmonary mucormycosis can closely resemble invasive pulmonary aspergillosis. If the infection is not contained at its primary site, it can lead to dissemination. A middle-aged woman, initially diagnosed with acute promyelocytic leukaemia, presented with atypical non-resolving pneumonia. Despite empirical antifungal treatment targeting Candida and Aspergillus, her clinical condition continued to deteriorate, resulting in the spread of the infection. Subsequent follow-up, combined with a high index of suspicion and isolation of Mucorales from an extrapulmonary site, identified mucormycosis as the causative pathogen. Early recognition of subtle clinical and radiological cues and prompt initiation of a classical medico-surgical approach is crucial for treating invasive mucormycosis and managing the underlying immunosuppressive condition. This case underscores the importance of early suspicion of Mucorales based on clinical and radiological signs, distinguishing it from invasive aspergillosis. The atypical presentation of pulmonary, splenic, and renal mucormycosis in this patient highlights the need for vigilance and timely intervention to prevent the most severe outcomes of disseminated mucormycosis.
KEYWORDS: Acute promyelocytic leukemia; Aspergillosis; Invasive fungal diseases; Mucor
After the second wave of the COVID-19 pandemic, there has been a significant increase in the incidence of mucormycosis cases. The injudicious use of corticosteroids during the pandemic, the rising prevalence of diabetes mellitus, and other immunocompromised conditions have contributed to this outbreak.1 Due to the high diversity of causative pathogens, variable clinical presentations, and often confusing imaging findings, early diagnosis of invasive mucormycosis (IM) poses a significant challenge for physicians. Additionally, the clinical and radiological similarities between IM and invasive aspergillosis (IA) and the lack of accurate diagnostic methods other than tissue diagnosis and histopathology further complicate the clinical scenario.2,3 The use of empirical antifungal therapy can alter the natural progression of the disease, leading to atypical but less aggressive presentations. However, it remains crucial as a timely life-saving measure.4
Here, we present a case of febrile neutropenia where the initial clinical and radiological features suggested an undistinguishable invasive fungal infection (IFI), initially treated as IA but later confirmed as IM.
CASE REPORT
an undistinguishable invasive fungal infection (IFI), initially treated as IA but later confirmed as IM.
CASE PRESENTATION
A 41-year-old woman, previously well until a year ago, presented with complaints of insidious onset and gradually progressive generalized weakness, accompanied by a low-grade, undocumented, intermittent fever over the past three months. These symptoms have restricted her from engaging in strenuous activities for the past three months and household activities for the past month. Recently, she began experiencing high-grade fever spikes with chills and rigors. Additionally, she reported dizziness upon standing up or sitting, confining her to bed for past weeks. She had intermittent episodes of non-progressive dry cough but no difficulty in breathing, chest pain, loss of appetite, weight loss, hemoptysis, bleeding manifestations, palpitations, or generalized body swelling.
Initially, she visited a local hospital where she was found to have severe anaemia and thrombocytopenia, leading to her referral to a higher centre for specialized management.
Upon arrival at the emergency department, the patient was conscious and oriented but appeared ill. She had severe pallor and bilateral symmetrical pitting pedal edema. Her vital signs were stable, without signs of rapid breathing or tachycardia. Abdominal examination revealed moderate splenomegaly. Respiratory examination indicated left-sided coarse crepitations with bilateral symmetrical chest expansion. Other systemic examinations were unremarkable.
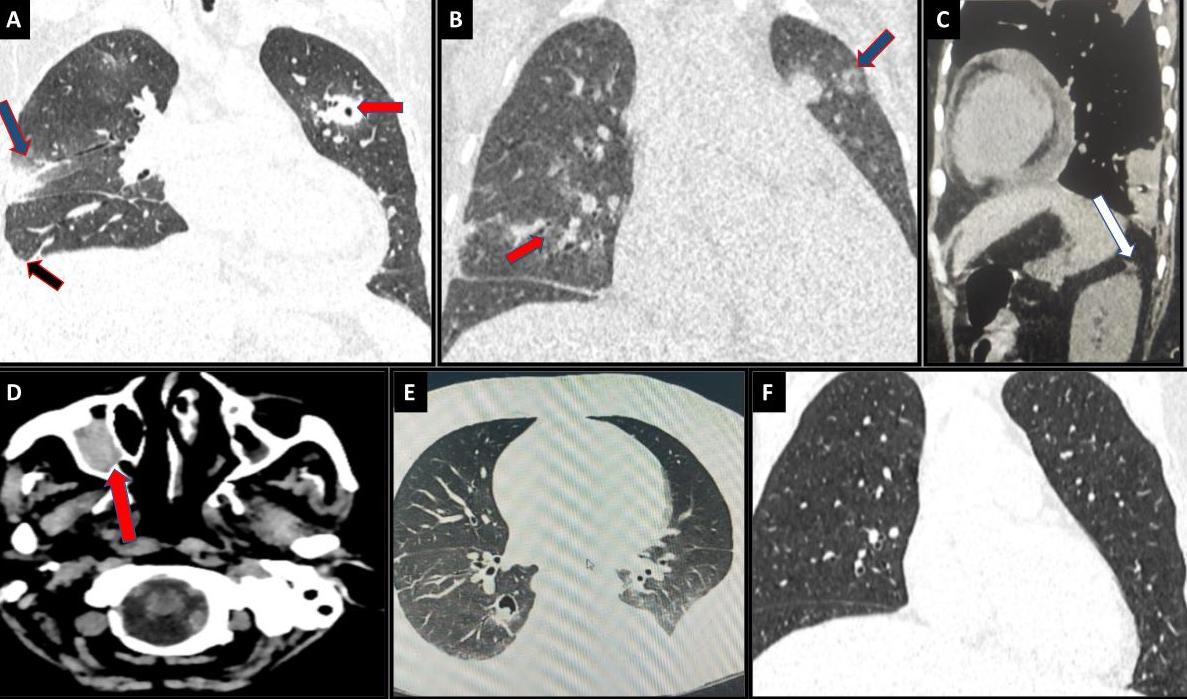
Her routine investigations revealed a haemoglobin level of 6 g/dL (normal range: 12-15 g/dL), a platelet count of 19 x 10³/μL (normal range: 150-400 x 10³/μL), and a total leukocyte count (TLC) of 4550 x 10³/μL (normal range: 4-11 x 10³/μL). Peripheral smear examination showed normocytic normochromic red blood cells with normal white blood cell counts but 95% blasts. The blast cells exhibited a high nucleus-to-cytoplasm ratio with 1-2 prominent nucleoli and strongly positive staining for myeloperoxidase. All markers for tropical fever were negative, and blood and urine cultures were sterile. A chest X-ray revealed opacities in the left mid and lower zones, while high-resolution computed tomography (HRCT) of the chest showed patchy areas of consolidation, ground-glass opacities (GGOs) in both lungs and a mild right-sided pleural effusion measuring 1.4 cm, suggestive of active infection (Figure 1A).
Figure 1. Radiological images of the thorax. (A) Coronal sections of high-resolution computed tomography Chest images done during the first visit to the hospital show patchy areas of ground glass opacity (blue arrow), cavitating nodules (red arrow), and Right-sided pleural effusion (black Arrow). Pleural effusions were more in favour of IM than IA. (B) 3D reconstruction HRCT images on follow-up visit (status post first consolidation with ATRA & ATO) showing active fungal lesions- cavitating nodules (red arrow) and fungal infiltrates (blue arrow). Note more than ten nodules are present, which is more favourable for mucormycosis. (C) Sagittal section from HRCT thorax with NCCT KUB suggesting mild perinephric fat stranding in the left kidney (HRCT was done one month before the onset of renal symptoms). (D) NCCT brain of the same patient suggestive of right maxillary sinusitis. (E) On treatment 3D-Reconstruction HRCT cuts showing multiple cavitary lesions and nodules in bilateral lungs; clearing of active fungal infiltrates in comparison to previous scans.. (F) Final Hrct showing residual cavity with no active infiltrates.
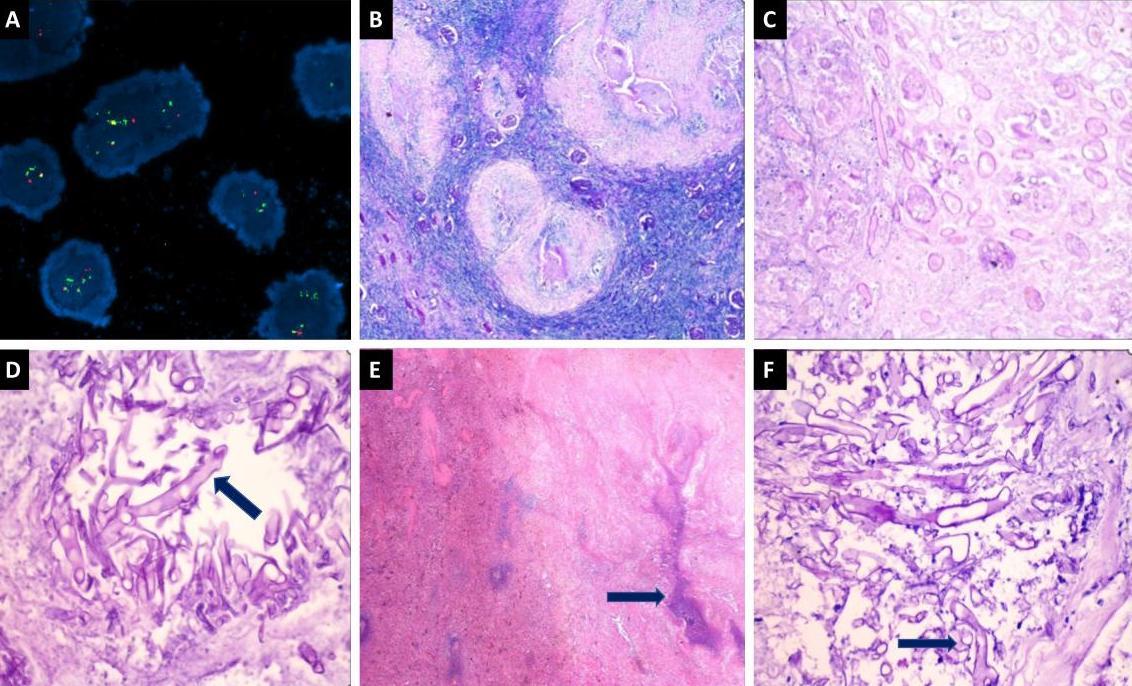
A bone marrow biopsy and fluorescence in situ hybridization testing confirmed the presence of the classic t(15:17) translocation (Figure 2). Consequently, she was diagnosed with acute promyelocytic leukaemia (APML), complicated by bacterial pneumonia, and was started on empirical antibiotics (piperacillin-tazobactam and azithromycin) and chemotherapy. Prophylactic antifungal treatment with posaconazole was also initiated. However, the pneumonia showed no clinical or radiological improvement, prompting consideration of fungal pneumonia. Although sputum, bronchoalveolar lavage (BAL) culture, and susceptibility tests were negative for Candida spp., caspofungin was initiated, given the high prevalence of Candida spp. in the epidemiological context.
Figure 2: Histopathological images of mucormycosis and FISH with PML RARA Positive. (A) Fluorescence in situ hybridisation (FISH) shows positive dual colour, dual fusion probe specific for PML/RARA (Promyelocytic Leukemia-Retinoic Acid Receptor Alpha) fusion [t(15;17)]. (B) Hematoxylin and eosin (H&E) sections from the kidney show few viable glomeruli along with large areas of necrosis (400X). (C) Higher magnification shows an area of necrosis along with fragmented broad aseptate fungal hyphae (200X). (D) Special stain for Periodic acid-Schiff (PAS) highlighting the mucormycosis fungal hyphae (400X). (E) H&E sections from the spleen show splenic parenchyma with large areas of necrosis (400X). (F): Higher magnification and special stain for PAS highlighting the mucormycosis fungal hyphae (400X).
The lack of response led to considering pulmonary aspergillosis, given the findings of air crescent sign, consolidations, and GGOs on HRCT and the patient’s underlying immunocompromised status. Due to technical constraints, beta-D-glucan, fungal polymerase chain reaction (PCR), and galactomannan tests could not be performed. However, IgG for Aspergillus fumigatus was negative.
The patient responded well to chemotherapy, achieving remission for APML soon after the first consolidation phase. After 33 days of All-Trans Retinoic Acid (ATRA) treatment and 18 days of Arsenic Trioxide (ATO) treatment, she became asymptomatic with stable blood counts. Caspofungin was discontinued after 21 days, and she was discharged following clinical recovery.
During a follow-up visit to the outpatient department one month after discharge, a repeat HRCT scan (Figure 1B) was performed, which showed similar findings in the chest. Old fungal infection changes were observed, along with an incidental finding of mild perinephric fat stranding in the left kidney (Figure 1C). The patient complained of mild, dull, aching left-sided flank pain without any Features of dysuria. Urine cultures were sterile, and urine for acid-fast bacilli staining was negative. She continued with ATO cycles and received empirical antibiotic treatment (nitrofurantoin) for the urinary tract infection. After completing the 4th cycle of ATO, the subsequent PML-RARA (Promyelocytic Leukemia-Retinoic Acid Receptor Alpha) report turned negative.
However, after another month, she presented with insidious onset, gradually progressive, mild to moderate intensity dull aching pain in the left lumbar and iliac region, lasting for one month. The pain radiated to the left flank and was accompanied by swelling in the left flank region, which gradually increased in size. The swelling was associated with reddish discolouration of the overlying skin and pain. She did not report decreased urine output, hematuria, frothy urine, or pus discharge in urine. There were no symptoms of generalized abdominal pain, vomiting, diarrhoea, blood in stools, constipation, shortness of breath, or palpitations. Upon local examination, a diffuse swelling measuring 8 X 4 cm² was observed in the left flank region. It was firm in consistency, had smooth surfaces, and exhibited focal tenderness. Abdominal ultrasonography revealed a heteroechoic lesion in the left renal fossa, suggesting a renal abscess extending to the spleen.
Guided aspiration from the renal abscess was performed using ultrasonography. Potassium hydroxide (KOH) mount examination of the pus revealed broad, aseptate hyphae with irregular branching at wide angles, and culture confirmed the presence of Mucorales. Fungal species identification and sensitivity could not be performed due to technical limitations. Routine investigations showed normal results, except for elevated HbA1c levels. The patient was initiated on liposomal amphotericin-B and scheduled for elective left nephrectomy with splenectomy. A preoperative CT scan of the thorax (Figure 1E) revealed an IFI with multiple nodules and a thick-walled cavitary area, displaying an air crescent sign in both lungs. The presence of pleural effusions, more than ten pulmonary nodules in CT scans, and sinus involvement observed in the non-contrast CT head sections (Figure 1D) supported the possibility of mucormycosis, albeit uncommon.
After obtaining pulmonology clearance, surgery was performed. During the postoperative period, multiple sputum samples, including bronchoalveolar lavage samples, were sent for fungal culture and KOH mount examination, all of which were negative. The patient continued to receive daily amphotericin-B and underwent frequent follow-ups to monitor the regression of pulmonary and residual renal lesions. The histopathology report of the nephrectomy and splenectomy confirmed the presence of mucormycosis in the spleen and kidney (Figure 2).
The patient’s residual renal and pulmonary lesions gradually improved in the one-month follow-up scan. After this, she was discharged and continued to be followed up in the outpatient department. Posaconazole injections were continued as part of the treatment regimen. During the four-month follow-up scan (Fig 1F), approximately 20% clearance of the pulmonary lesions was observed. Currently, she is receiving biweekly amphotericin-B injections and undergoing regular follow-up, with plans to monitor her progress and response to treatment to adjust the therapeutic approach as needed.
DISCUSSION
A study conducted in India revealed four significant clusters of invasive fungal infections: invasive candidiasis (20.9%), cryptococcosis (13.4%), invasive aspergillosis (40.7%), and mucormycosis (24.5%).5 Based on epidemiological data and the patient’s clinical presentation—including immunocompromised status, recurrent blood transfusions, hematologic malignancy, presence of an in situ central venous catheter, and use of broad-spectrum antibiotics—there was suspicion of an active IFI, particularly invasive candidiasis. Consequently, despite the absence of microbiologic evidence, the treatment was upgraded from prophylactic posaconazole to empirical caspofungin targeting Candida spp.
However, the lack of clinical and minimal radiological improvement observed after the 21-day course of caspofungin led to a retrospective analysis. The initial high-resolution computed tomography (HRCT) findings, such as GGOs and airspace consolidations, were nonspecific but could be seen in pulmonary candidiasis. Nevertheless, the overall clinical and radiological response ‘to treatment did not indicate Candida spp. infection. Considering the limited efficacy of caspofungin and the absence of microbiologic evidence, the suspicion of other IFIs causing cavitary pneumonia became more prominent. This retrospective analysis and the lack of response to caspofungin prompted a re-evaluation of the diagnosis and management approach for the patient’s condition. Based on the clinical-radiological features, the pathogenesis mainly pointed to IM and IA.
Mucormycosis, a fungal infection caused by various fungi of the order Mucorales, poses diagnostic challenges due to the diverse evasion mechanisms employed by these fungi against the host immune system and their similarities with other fungi causing IFI.6-8 Both IM and IA share comparable disease pathogenesis, risk factors, clinical presentations, and radiological signs, albeit with subtle distinguishing features. IM is often linked with acute leukaemias (such as AML and ALL) and graft-versus-host disease, post-hematopoietic stem cell transplantation. In contrast, IA tends to be more prevalent in lymphomas. However, risk factors for IFI are not mutually exclusive. IM presents unique risk factors like diabetes, iron overload, and burns.
Clinical presentations of mucormycosis vary depending on the site of infection, with rhino-orbital-cerebral mucormycosis being the most common, followed by cutaneous, pulmonary, and disseminated forms.9 Disseminated mucormycosis, involving multiple non-continuous organ systems, often presents with renal involvement, either as part of haematological dissemination or rarely as isolated renal mucormycosis. Dissemination typically originates from primary sites like the sinus mucosa and alveoli.10,11 Pulmonary involvement is more typical in IA, while mucormycosis commonly involves the paranasal sinuses (PNS) and exhibits dissemination (72% in IM vs. 5% in IA). Clinical manifestations also differ, with pulmonary mucormycosis more frequently associated with haematological malignancies, whereas rhino-cerebral mucormycosis is more common in diabetic patients.10,12 In the presented case, the patient primarily presented with pulmonary involvement due to acute promyelocytic leukaemia (APML), raising suspicions of both IA and IM. Considering the possibility of disseminated mucormycosis in a diabetic and neutropenic patient involving the PNS, non-contract computed tomography (NCCT) sinuses was performed despite the absence of primary sinusitis symptoms. The subsequent identification of dense opacification in the right maxillary sinus played a pivotal role in shifting the diagnostic focus toward IM.
Radiological differentiation between IM and IA can pose challenges due to shared features. Pulmonary nodules, ground-glass opacities (such as the halo sign, observed in approximately 21% of IM cases and 25% of IA cases), and the air crescent sign can manifest in both conditions. However, mucormycosis tends to be associated with a higher incidence of pleural effusion, destructive lesions, the presence of more than ten lung nodules, and the reverse halo sign (noted in approximately 19% of IM cases compared to less than 1% in IA).10,13-16
Advancements in radiological imaging have made early detection of IFIs more feasible. However, this early detection also presents challenges in differentiating between various IFIs based on nonspecific early radiological findings. In the present case, a CT scan revealed significant findings favouring the diagnosis of IM over IA, primarily due to pleural effusion, destructive lesions, multiple lung nodules, and other characteristic features associated with mucormycosis.
When diagnosing mucormycosis, the availability of biomarkers aiding in noninvasive diagnosis is limited compared to invasive candidiasis and aspergillosis, which have the beta-d-glucan assay and galactomannan test, respectively.17-18 Microscopy of bronchoalveolar lavage (BAL) samples exhibit low sensitivity in both aspergillosis (approximately 50%) and pulmonary mucormycosis (<40%).19 Therefore, the gold standard for diagnosing IA and IM remains tissue sampling and histopathological examination, where fungal hyphae and angioinvasion confirm the diagnosis. Fungal hyphae in KOH mount have characteristic findings to differentiate Aspergillus vs. Mucorales, respectively: 1) width – narrower vs broad, 2) septation – septate vs non-septate, 3) branching – regular at acute angles vs irregular at right angles, and 4) appearance – uniform vs twisted and irregular appearance. In this case, the possibility of Aspergillus-Mucorales co-infection, although rare, was ruled out during the histopathological examination of fungal hyphae in the kidney and spleen.
The decision not to perform lobectomy in the lungs was made considering the patient’s immunocompromised state, prior major surgery, and ongoing anti-cancer drug treatment. Despite being in the remission phase of acute promyelocytic leukaemia (APML), the patient’s overall condition and potential risks associated with major lung surgery were taken into account.
IM is a highly devastating disease, with estimated mortality rates in patients with malignancies reaching 66%.11 Mortality rates can vary depending on the site of infection and the patient’s immune status, with the highest mortality observed in disseminated cases (96%) and the lowest in cases involving the sino-nasal region (46%), even after appropriate medical and surgical treatment.11 In the presented case, although the patient was initially immunocompromised, the simultaneous management of APML along with proper antifungal coverage likely contributed to the patient’s recovery from mucormycosis despite its known indolent course in immunocompetent individuals.20
Early histopathology and culture are crucial for diagnosing any IFIs. In a resource-rich scenario, pan-fungal PCR, liquid biopsy, and various serological markers can be relied upon. In the current era, with significant improvements in survival rates for neutropenic patients, another complication encountered is the co-infection of various IFIs. Hence, the suggested approach includes early tissue diagnosis, frequent re-evaluation of the diagnosis, and ensuring appropriate treatment in any case of suspected IFI.
CONCLUSION
Clinical and radiological presentations can often mislead toward IA diagnosis. Especially when BAL and sputum samples yield poor sensitivity, invasive tissue diagnosis remains paramount. Any patient exhibiting potential signs of IA with lung involvement should prompt consideration of IM if there is concurrent renal or spleen involvement, suggesting dissemination. Disseminated IM may include asymptomatic sinus involvement. Therefore, non-contrast CT of the sinuses should be performed in all suspected cases. Successful management of disseminated IM entails a multidisciplinary approach, integrating medical and surgical interventions tailored to address the underlying immunocompromised conditions.
INFORMED CONSENT
Written informed consent was obtained from the patient. Confidentiality of the patient was maintained in the article.
CONFLICTS OF INTEREST STATEMENT
The authors declare no conflict of interest.
SOURCE OF FUNDING
None
AUTHOR’S CONTRIBUTION
SV: Data collection; Analysis; Writing the draft
RHP: Data collection; Analysis; Writing the draft
PKP: Conceptualization; Investigation; Methodology; Resources; Review
VKP: Supervision; Validation; Review & Editing
REFERENCES
1. Ahmadikia K, Hashemi SJ, Khodavaisy S, et al. The double-edged sword of systemic corticosteroid therapy in viral pneumonia: A case report and comparative review of influenza-associated mucormycosis versus COVID-19 associated mucormycosis. Mycoses. 2021;64(8):798-808.
2. Wang Q, Liu B, Yan Y. Disseminated mucormycosis (DM) after pneumonectomy: a case report. BMC Infect Dis. 2016;16:337.
3. Yan D, Dong H, Shan B. Progress of epidemiology and diagnosis of invasive fungal infections. Progress of Microbiology Immunology. 2009;37(1):67–71.
4. Reddy GKK, Padmavathi AR, Nancharaiah YV. Fungal infections: Pathogenesis, antifungals and alternate treatment approaches. Curr Res Microb Sci. 2022;3:100137.
5. Klastersky J. The changing face of febrile neutropenia-from monotherapy to moulds to mucositis. Why empirical therapy?. J Antimicrob Chemother. 2009;63 Suppl 1:i14-i15.
6. Webb BJ, Ferraro JP, Rea S, Kaufusi S, Goodman BE, Spalding J. Epidemiology and Clinical Features of Invasive Fungal Infection in a US Health Care Network. Open Forum Infect Dis. 2018;5(8):ofy187.
7. Nicolás FE, Murcia L, Navarro E, Navarro-Mendoza MI, Pérez-Arques C, Garre V. Mucorales Species and Macrophages. J Fungi (Basel). 2020;6(2):94.
8. Oren I, Paul M. Up to date epidemiology, diagnosis and management of invasive fungal infections. Clin Microbiol Infect. 2014;20 Suppl 6:1-4.
9. Jeong W, Keighley C, Wolfe R, et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019;25(1):26-34.
10. Klimko N, Khostelidi S, Shadrivova O, et al. Contrasts between mucormycosis and aspergillosis in oncohematological patients. Med Mycol. 2019;57(Supplement_2):S138-S44.
11. Chakrabarti A, Dhaliwal M. Epidemiology of mucormycosis in India. Curr Fungal Infect Rep. 2013;7(4):287–92.
12. Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634-53.
13. Hernández JL, Buckley CJ. Mucormycosis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024.
14. Georgiadou SP, Sipsas NV, Marom EM, Kontoyiannis DP. The diagnostic value of halo and reversed halo signs for invasive mold infections in compromised hosts. Clin Infect Dis. 2011;52(9):1144-55.
15. Weissleder R, Wittenberg J, Harisinghani MG, Chen JW. Primer of Diagnostic Imaging. 5th ed. St. Louis, Missouri: Mosby; 2011.
16. Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34(7):909-17.
17. Dabas Y, Xess I, Pandey M, et al. Epidemiology and Antifungal Susceptibility Patterns of Invasive Fungal Infections (IFIs) in India: A Prospective Observational Study. J Fungi (Basel). 2021;8(1):33.
18. Lee FY, Mossad SB, Adal KA. Pulmonary mucormycosis: the last 30 years. Arch Intern Med. 1999;159(12):1301-9.
19. Kelly BT, Pennington KM, Limper AH. Advances in the diagnosis of fungal pneumonias. Expert Rev Respir Med. 2020;14(7):703-14.
20. Das A, P AK, Panda PK, Gupta AK. Cerebral Mucormycosis without Rhino-Orbital Involvement in a Patient with Chronic Kidney Disease. JASPI. 2023;1(1):34–7.
Submit a Manuscript:
Copyright © Author(s) 2024. JASPI- Journal of Antimicrobial Stewardship Practices and Infectious Diseases.

