Non-tuberculous Mycobacteria Osteomyelitis of Patella with Prepatellar Bursitis of Knee
JASPI September 2024/ Volume 2/Issue 3
Copyright: © Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Garg A, Aggarwal A, Vishwajeet V, Samantaray S. Non-tuberculous Mycobacteria Osteomyelitis of Patella with Prepatellar Bursitis of Knee. JASPI. 2024;2(3):-29-34 DOI: 10.62541/jaspi038
ABSTRACT
Tuberculosis has been persisting with mankind since time immemorial, which delineates its ubiquitous nature. However, infections with non-tubercular mycobacteria of musculoskeletal tissue are rare. Though the knee joint is the third most common site of involvement after the spine and hip, atypical mycobacterial infection of the patella in an immunocompetent patient is unheard of. Herein, we present a case of a late adolescent male with diffuse painful swelling of the left knee, which came to be diagnosed as Non-tuberculous Mycobacteria osteomyelitis of the patella left side for its rarity and its successful management by antitubercular chemotherapy without any surgical intervention. High suspicion, early diagnosis and anti-NTM chemotherapy bring satisfactory outcomes.
KEYWORDS: Acid-fast bacilli, bursitis, immunocompetent, knee, Mycobacterium fortuitum, osteomyelitis
INTRODUCTION
Non-tuberculous Mycobacteria (NTM) includes more than 120 mycobacterial species other than Mycobacterium tuberculosis and Mycobacterium leprae. Although NTM is distributed in the environment and cultured from soil and lake, they can infect immunocompromised patients such as those who have HIV, cystic fibrosis, and organ transplant recipients and quite rarely infect immunocompetent individuals.1 The disease spectrum of bone and joint NTM infections varies according to the immune status of the individual, manifesting as focal or localized in immunocompetent individuals to multifocal and disseminated in immunocompromised.2 In a study published in 2022, 45% of NTM infections were reported in immunocompetent hosts from a total of 118 cases recorded in a seven-year retrospective study.3 American Society of Clinical Pathology in 2007 depicted the prevalence of Mycobacterium fortuitum as 28.7% in the cohort of 115 cases.4 In contrast, the study in 2022 by Chai et al. identified a total of 3.4% cases of M. fortuitum.3 According to one research, 60% have been classified as opportunistic human pathogens and have the ability to initiate diseases.5
The dilemma of early and accurate diagnosis amid the indolent course of the disease, the difficulty of culture, and the isolation of the causative pathogen species and its drug sensitivity remains a challenge for clinicians and microbiologists to date. Although immunocompromised patients are the primary sufferers of NTM, normal hosts are no longer the exception, as exemplified in this case. This adds to the confusion in diagnosing the disease early, thus delaying the treatment and increasing the morbidity and mortality database.
CASE PRESENTATION
A late adolescent male presented to the outpatient department of a tertiary care centre with a complaint of swelling and pain in the left knee for three months. On questioning, there was a history of blunt trauma to the knee, Patient did not present with any history of fever only a local rise of temperate. Nno history of weight loss or loss of appetite ruling out neoplastic etiology.
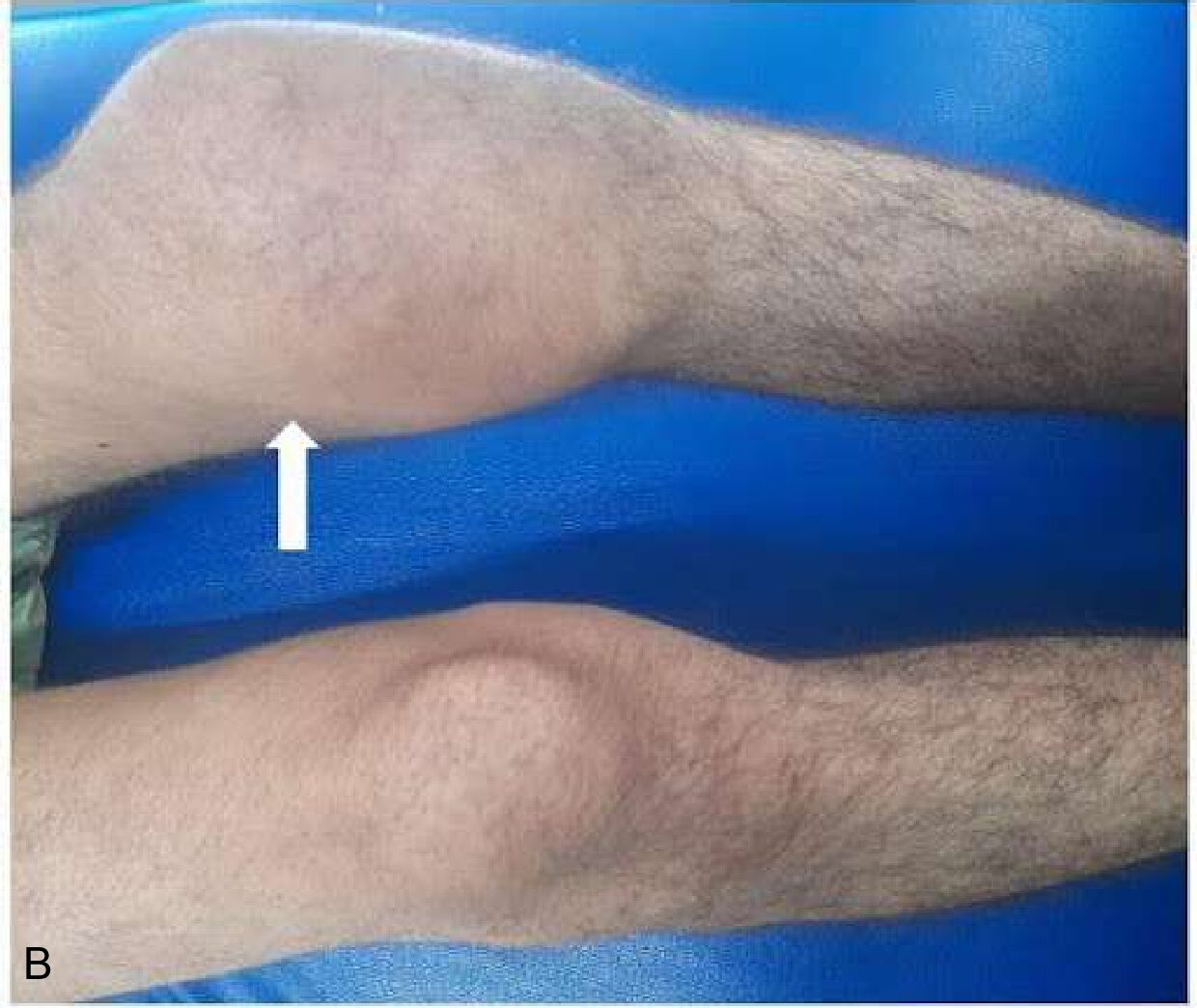
Physical examination (Figure 1A) reveals tenderness at the knee and supra patellar region, local rise of temperature, decrease in range of motion of the knee from forty to ninety degrees and swelling at the suprapatellar and prepatellar region without neurovascular deficit. There was a fixed flexion deformity of 40 degrees, and the patient had difficulty walking.
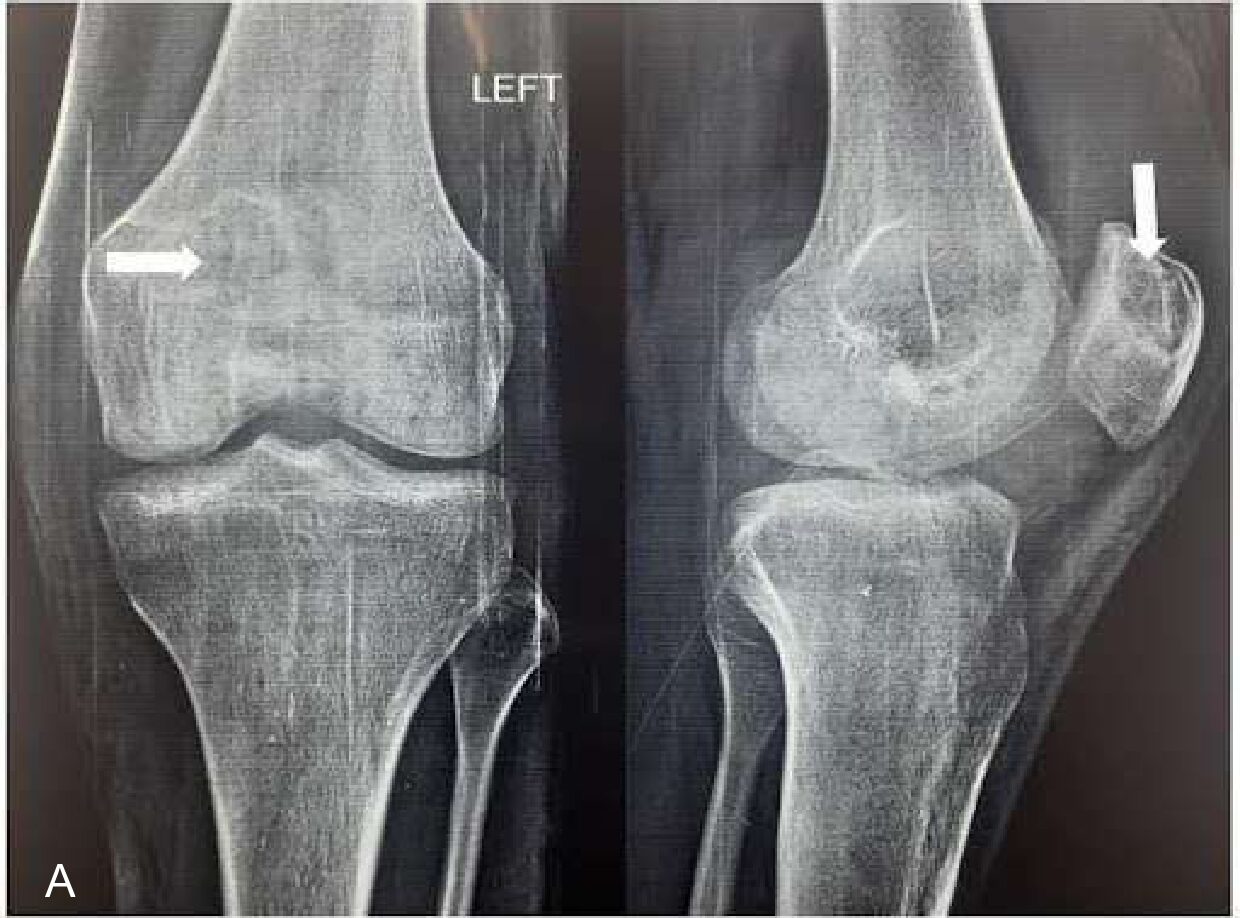
Roentgenographic examination reveals a lytic lesion at the superior pole of the left patella and increased prepatellar and supra-patellar soft tissue density (Figure 1B). Ultrasonography showed significant joint effusion with intramuscular oedema and collection pockets in anterior muscles above and below the knee
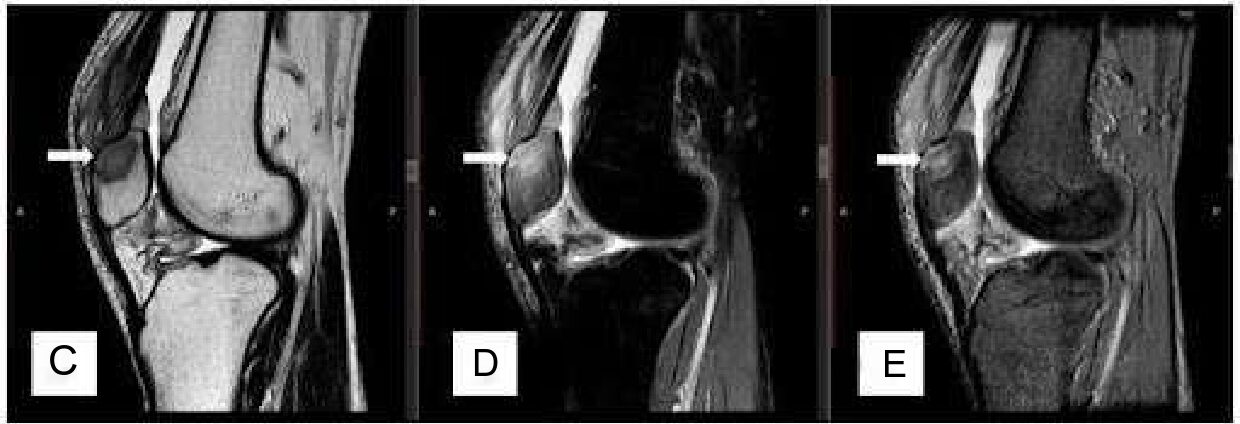
joint. Blood investigations reveal lymphocytosis (4,200 cells/microliter) and increased ESR (80 mm in the first hour), and hs-CRP levels were 105 mg/l (normal range 0.5–10 mg/l), Haemogram- Hb was found 12.3gm, Platelet was 1.80 Lakh/ml, LFT & RFT were within normal Range. Magnetic resonance imaging (MRI) suggested acute focal osteomyelitis of the superior pole of the patella with prepatellar bursitis and synovitis of the left knee (Figure 1C-E).
Figure 1. Clinico-radiological images of the left knee. (A) showing tense swelling and redness obliterating the suprapatellar pouch and infrapatellar fossae (marked with white arrow) compared to the contralateral side; (B) showing a roentgenographic image of left knee anterior-posterior and a lateral view showing lytic lesion (marked with white arrow) at the superior pole of the patella; (C) showing MRI T1w image of knee joint sagittal view showing hypo intensity at the superior pole of the patella with prepatellar bursitis and synovitis of the left knee; (D) showing MRI 2D FS-PD image showing hyperintensity at the superior pole of patella with denudation of the cartilage due to bony necrosis; (E) showing MRI T2w image showing hyperintensity at the superior pole of the patella (lesions shown with white arrow).
Knee aspirate for synovial fluid analysis showed marked cellularity with lymphocytosis. Microscopic Ziehl-Neelsen staining showed beaded acid-fast bacilli (AFB) (Figure 2A). Based on the patient’s constitutional symptoms and smear positivity, standard treatment with anti-tubercular drugs was started.
Synovial fluid culture for mycobacteria by automated method flagged positive after eight days. A smear prepared from the broth culture was positive for acid-fast bacilli, but the MPT-64 antigen test (SD BIOLINE) was negative. GeneXpert (CBNAAT) did not detect M. tuberculosis. The isolate obtained was identified as Mycobacterium fortuitum by Line Probe assay (LPA). Considering the above microbiological findings, a diagnosis of Non-tubercular or Atypical mycobacterial infection of the patella was made.
Computed Tomography (CT) guided core needle biopsy was obtained from the lesion at the superior pole of the patella and sent for histopathological examination, which showed necrosis with acute inflammation and dead bony spicules (Figures 2B-C). Microscopy evaluation of the biopsy sample had findings similar to synovial fluid findings. NTM, ubiquitous, usually has a doubtful pathological significance unless it meets the criteria of either repeated isolation from the same patient or isolation from sterile body fluid along with supportive clinical and radiological evidence5. Based on the above clinical presentation, microbiological reports and histopathological images, the final diagnosis of non-tubercular osteomyelitis of patella with prepatellar bursitis was made after repeated isolation.
Infectious disease specialist consultation was sought, after which the patient was started on triple-drug therapy, including injectable amikacin (500mg IV OD), injectable imipenem (1g IV BD) and linezolid tablet (600mg OD) for period of six months.
On the assessment of the patient, six months post-treatment, the pain and swelling of the knee subsided. The knee range of motion improved with the commencement of the physiotherapy exercises. A repeat radiograph revealed sclerosis of the lytic lesion of the patella (Figure 2D). The patient was advised to continue the oral regimen for another six months.
Figure 2. Micro-radio-histopathological images of the left knee. (A) showing microscopic Ziehl-Neelsen staining suggestive of long cylindrical acid-fast bacilli (AFB) (1000x magnification) (Marked with a black arrow); (B) showing Computed Tomography (CT) guided core needle biopsy taken from the lesion at the superior pole of the patella with the help of a Jamshidi trephine needle, showing 3-4 long cylindrical cores (shown with white arrow); (C) showing histopathological examination with the magnification of 10x showing necrosis and dead bony spicules and 40x microscopy showing red bacilli within necrosed trabeculae (picture in picture, with black arrow); (D) showing roentgenographic image of left knee anterior-posterior and a lateral view showing sclerosis (marked with white arrow) at the superior pole of the patella after antitubercular chemotherapy.
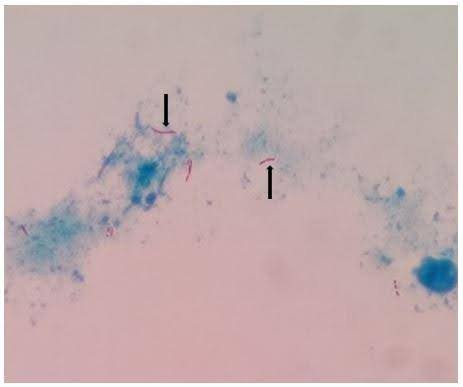
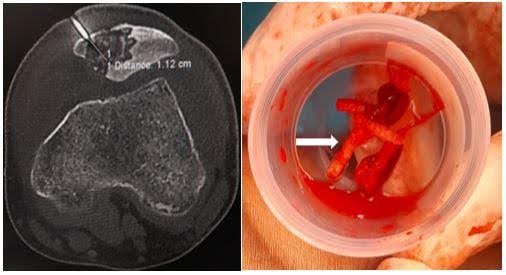
Fig. [A] Fig.[B]
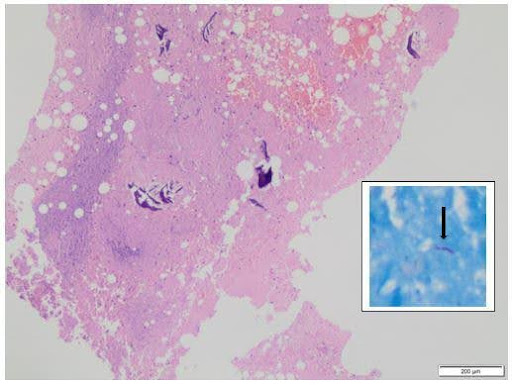
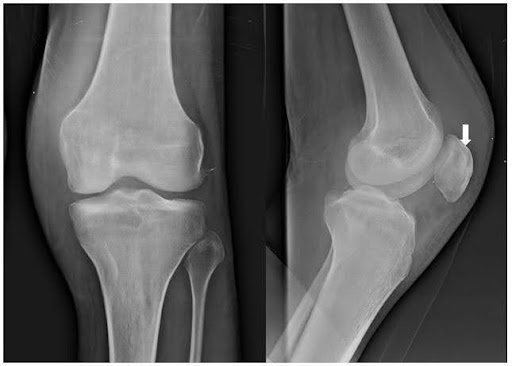
Fig.[C] Fig.[D]
DISCUSSION
The year 1950 proved to be the benchmark when NTM was recognized as a human pathogen.6 Since then, more than 125 species have been recognized, of which 60 are known to cause clinical manifestations in humans.7 Atypical mycobacterial infections in the musculoskeletal system are usually opportunistic in nature and invade those belonging to vulnerable categories such as HIV infection, leukaemia, lymphoma and immunosuppressive drugs. Studies have found rapidly growing mycobacteria such as M. fortuitum, M. abscesses and M. chelonae to be the more common NTM species associated with immunocompetent individuals. On the other hand, species like M. marinum and M. hemophilum are recovered from immunocompromised individuals.6,8
Among the common NTM species causing osteoarticular infections, M. fortuitum, M. chelonae, and M. marinum are often acquired by trauma (as seen in M. ulcerans following snake bite or M. abscessus as in injection drug abuser) or previous surgical intervention, which is usually the common risk factor in immunocompetent host.9–12 Cases of osteomyelitis can occur even in the absence of any penetrating injury, disseminated disease or any immunosuppression. In the above-stated cases, any blunt trauma can lead to active infection following the principle of locus minoris resistentiae,12 wherein blunt trauma reveals a vulnerable area prone to seeding of bacilli via the macrophages, causing the release and activation of dormant mycobacteria, initiating a new focus of infection This case reports NTM infection of the patella in immunocompetent patient following trauma. Contrary to the fact that the hand or wrist is considered to be the most frequent site of infection,11 in our case, the patient had superior pole of patella involvement, which has not been reported so far.
In 2014, Scarparo et al. delineated from a study done on 29 patients that the mean duration for diagnosis of musculoskeletal NTM infection was approximately ten months.11 In our case, microscopic Ziehl-Neelsen staining was suggestive of AFB, and automated culture after eight days was AFB positive and rapid MPT 64 antigen test negative.13 Also, CBNAAT was negative, which aroused high suspicion of NTM infection, which LPA confirmed. With the advent of CBNAAT, the duration of the diagnosis has drastically decreased.
The main challenge while diagnosing these kinds of infections is the availability of molecular diagnostic techniques (such as Line probe assay), which are usually available at tertiary care centers and can differentiate and speciate among atypical mycobacteria.
India’s status as the world’s largest tuberculosis epidemic, coupled with a lack of heightened awareness regarding NTM infections and their diagnosis, maintaining a high index of suspicion is the key to managing such cases and restricting the inadvertent use of empirical antibiotics, which only adds to drug resistance. Without the commencement of any empirical antibiotic for the lytic lesion of bone as seen on the radiograph, keeping in mind the endemicity of tuberculosis, we did a workup to rule out tuberculosis of the patella as the first thing that had not been reported in such a presentation earlier.
As per ATS (American Thoracic Society)/ IDSA (Infectious Diseases Society of America),14 NTM osteomyelitis can occur through a hematogenous route and explain the rationale behind the patients having NTM infection of axial skeleton and extremities without any external source of injury and can occur in patients with blunt trauma which is being exemplified in our case. Clinically, musculoskeletal NTM infections basically resemble those manifestations which are caused by Mycobacterium tuberculosis, although the gross course of the NTM osteomyelitis is usually milder as compared to that of conventional Mycobacterium tuberculosis.15
There is no standardized treatment regime for the management of NTM osteomyelitis.16 Factors such as infection site, extent of disease, species, and drug response collectively guide the duration of the medication, which is tailor-made for each patient accordingly.
A combination of three drugs is followed to manage NTM infection, as monotherapy can lead to the development of resistance. NTM are usually intrinsically resistant to antitubercular drugs isoniazid, ethambutol and rifampicin.14 Macrolides-based drug regime is promising to some extent as far as results are concerned. Still, its activity varies on the presence of the erm gene, which confers inducible resistance to macrolides.7,17 Rapidly growing mycobacteria have shown in vitro susceptibility to amikacin, carbapenems (imipenem and meropenem), oxazolidinones (linezolid and tedizolid) and clofazimine.17 In our case, we also include imipenem along with amikacin in the treatment apart from linezolid as a three-drug regime, to which the patient responds quite satisfactorily for the duration of one year. Treatment duration recommended in the case of vertebral diseases is 12 months, whereas six to nine months of drug therapy for other bone diseases is preferred.18,19
CONCLUSIONS
Musculoskeletal NTM infection is rare, and osteomyelitis of the patella due to atypical mycobacterium in immunocompetent patients is unknown. Blunt trauma can also result in the commencement of infection. High suspicion, early diagnosis and anti-NTM chemotherapy may result in satisfactory outcomes. Usually, six to twelve months of antitubercular will suffice with or without surgical intervention, depending on the patient’s condition.
INFORMED CONSENT
Written informed consent was obtained from the patient. Confidentiality of the patient was maintained in the article.
CONFLICTS OF INTEREST STATEMENT
The authors declare no conflict of interest.
SOURCE OF FUNDING
None
AUTHOR’S CONTRIBUTION
AG: Conceptualization; Writing the draft
AA: Investigation; Methodology; Supervision; Review & Editing
VV: Investigation; Methodology
SS: Validation; Review & Editing
REFERENCES
Petitjean G, Fluckiger U, Schären S, Laifer G. Vertebral osteomyelitis caused by non-tuberculous mycobacteria. Clin Microbiol Infect. 2004;10(11):951-3.
Holt MR, Kasperbauer S. Management of Extrapulmonary Non-tuberculous Mycobacterial Infections. Semin Respir Crit Care Med. 2018;39(3):399-410.
Chai J, Han X, Mei Q, et al. Clinical Characteristics and Mortality of Non-tuberculous Mycobacterial Infection in Immunocompromised vs. Immunocompetent Hosts. Front Med (Lausanne). 2022;9:884446.
Han XY, Dé I, Jacobson KL. Rapidly growing mycobacteria: clinical and microbiologic studies of 115 cases. Am J Clin Pathol. 2007;128(4):612-21.
Samaddar A, Srivastava S, Khan S, et al. Mycobacterium chelonae bacteremia in a patient with myasthenia gravis receiving long-term steroid therapy. Access Microbiol. 2019;1(10):e000069.
Theodorou DJ, Theodorou SJ, Kakitsubata Y, Sartoris DJ, Resnick D. Imaging Characteristics and Epidemiologic Features of Atypical Mycobacterial Infections Involving the Musculoskeletal System. Am J Roentgenol. 2001;176(2):341-9.
Bi S, Hu FS, Yu HY, et al. Non-tuberculous mycobacterial osteomyelitis. Infect Dis. 2015;47(10):673-85.
Wong KP, Tang ZH, Tan GM. Mycobacterium fortuitum and Mycobacterium abscessus infections in the foot and ankle in two immunocompetent patients. BioMedicine. 2020;10(4):52-6.
Gundavda MK, Patil HG, Agashe VM, Soman R, Rodriques C, Deshpande RB. Non-tuberculous mycobacterial infection of the musculoskeletal system in immunocompetent hosts. Indian J Orthop. 2017;51(2):205-12.
Wani SR, Wattal C, Raveendran R. Epidemiology and risk factors associated with NTM pulmonary and extrapulmonary infections in a high tuberculosis endemic Region. Indian J Med Microbiol. 2020;38(2):169-75.
Piersimoni C, Scarparo C. Extrapulmonary Infections Associated with Non-tuberculous Mycobacteria in Immunocompetent Persons. Emerg Infect Dis. 2009;15(9):1351-8.
Chan ED, Kong PM, Fennelly K, Dwyer AP, Iseman MD. Vertebral Osteomyelitis Due to Infection with Non-tuberculous Mycobacterium Species after Blunt Trauma to the Back: 3 Examples of the Principle of Locus Minoris Resistentiae. Clin Infect Dis. 2001;32(10):1506-10.
Kanade S, Nataraj G, Suryawanshi R, Mehta P. Utility of MPT 64 antigen detection assay for rapid characterization of mycobacteria in a resource-constrained setting. Indian J Tuberc. 2012;59(2):92-6.
Griffith DE, Aksamit T, Brown-Elliott BA, et al. An Official ATS/IDSA Statement: Diagnosis, Treatment, and Prevention of Non-tuberculous Mycobacterial Diseases. Am J Respir Crit Care Med. 2007;175(4):367-416.
Wolinsky E. Mycobacterial Diseases Other Than Tuberculosis. Clin Infect Dis. 1992;15(1):1-12.
Moritake A, Mori S, Kamiya M, et al. Non-tuberculous mycobacterial infection of the knee after arthrocentesis for idiopathic hemarthrosis: A case report. Ann Med Surg. 2021;65:102332.
Hatakeyama S, Ohama Y, Okazaki M, Nukui Y, Moriya K. Antimicrobial susceptibility testing of rapidly growing mycobacteria isolated in Japan. BMC Infect Dis. 2017;17:197.
Sharma SK, Upadhyay V. Epidemiology, diagnosis & treatment of non-tuberculous mycobacterial diseases. Indian J Med Res. 2020;152(3):185-226.
Hogan J, Rm H, Sb N. Mycobacterial Musculoskeletal Infections. Infect Dis Clin North Am. 2017;31(2):369-82.
Submit a Manuscript:
Copyright © Author(s) 2024. JASPI- Journal of Antimicrobial Stewardship Practices and Infectious Diseases.

