Persistent Candidemia in the Postpartum Period: A Case of Uterocutaneous Fistula and the Role of Dual Antifungal Therapy
JASPI September 2024/ Volume 2/Issue 3
Copyright: © Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Prakash J, B A, Sharma T.Persistent Candidemia in the Postpartum Period: A Case of Uterocutaneous Fistula and the Role of Dual Antifungal Therapy. JASPI. 2024;2(3):-35-39 DOI: 10.62541/jaspi029
ABSTRACT
Candidemia, the systemic spread of Candida spp, is a significant concern in intensive care units and among immunocompromised patients. This case report details a young postpartum female who developed persistent candidemia following a lower-segment cesarean section. Postoperatively, she experienced a high-grade fever, respiratory distress, and disorientation, initially managed with broad-spectrum antibiotics and later with antifungal therapy. Blood cultures confirmed Candida glabrata, and imaging revealed a likely útero-cutaneous fistula as the infection source. Despite initial monotherapy, the patient showed no improvement, prompting the addition of a second antifungal agent. The dual regimen of caspofungin and voriconazole led to clinical improvement, and subsequent blood cultures were sterile. The patient was discharged on oral fluconazole. This case underscores the complexities of managing persistent candidemia, particularly in the postpartum period, and highlights the potential benefits of dual-antifungal therapy in refractory cases. Comprehensive imaging and a multidisciplinary approach were crucial in identifying and managing the uncommon source of infection. This report contributes to the limited literature on postpartum candidemia and emphasizes the need for thorough evaluation and tailored treatment strategies. Further research is needed to establish clear guidelines for using combination antifungal therapy in persistent candidemia and to explore the management of complex cases involving uncommon sources of infection.
KEYWORDS: Candida glabrata; Candidemia; Invasive candidiasis; Postpartum infection
INTRODUCTION
Candidemia, the systemic spread of Candida spp, is prevalent in intensive care units (ICUs) and among immunocompromised patients.1 Clinical manifestations can range from mild fever to septic shock, complicating differentiation from bacterial sepsis. Hematogenous dissemination may lead to invasive candidiasis, affecting multiple organs and potentially resulting in failure.2 Though rare, Candida pneumonia should be considered in patients unresponsive to standard treatments.3 While candidal vulvovaginitis is common in the third trimester and requires treatment to prevent dissemination,4 candidal sepsis in the postpartum period is uncommon.5
Advances in imaging have improved the detection and diagnosis of intra-abdominal candidiasis.6 Treatment includes prompt initiation of antifungal therapy such as echinocandins, azoles, and amphotericin B formulations.7 The bedside Candida score aids in identifying patients needing treatment, considering factors like severe sepsis, shock, total parenteral nutrition (TPN), catheters, invasive ventilation, dialysis, and immunosuppression.8 Susceptibility testing for azoles and echinocandins is recommended for all clinically significant Candida isolates.9 Non-neutropenic patients with candidemia are typically started on an echinocandin.2 When patients are clinically stable, with fluconazole-susceptible Candida isolates and negative blood cultures, a switch to oral fluconazole is suggested.10
This case report is notable for identifying invasive candidiasis in the postpartum period, with the patient ultimately responding to a dual-antifungal regimen.
CASE PRESENTATION
A 26-year-old female from Bihar, India, underwent a lower segment cesarean section (LSCS), after which she developed a fever and was treated for a suspected intra-abdominal infection in an ICU setting. She was discharged after initial clinical improvement. However, the fever recurred shortly afterward, characterized by intermittent high-grade spikes and accompanied by chills. There were no associated symptoms such as headache, rigors, joint pain, or rash. Additionally, the patient complained of shortness of breath for the past two days, which was sudden in onset, worsened upon lying down, but improved partially when sitting up. There was no history of decreased urine output or yellowish skin discoloration. The patient had no history of altered sensorium, bleeding manifestations, abdominal pain, vomiting, or diarrhoea. In her past medical history, she had undergone LSCS, and she had a prior episode of seizure approximately one month prior, potentially related to eclampsia. She had no history of hypertension, type 2 diabetes mellitus, tuberculosis, or asthma.
On examination, the patient was conscious but disoriented. The Glasgow Coma Scale (GCS) score suggested mild impairment. Her vital signs included a blood pressure of 144/80 mmHg, heart rate of 116 beats/minute, and oxygen saturation of 98% on 15 liters/min oxygen with a non-rebreather mask. Jugular venous pressure was not increased, but pallor and icterus were present. There was no cyanosis or clubbing. Bilateral pitting pedal edema was noted. A central line was in place in the right internal jugular vein for the past month.
Respiratory examination revealed equal air entry bilaterally with bilateral fine crepitations. Cardiovascular examination revealed normal heart sounds with no murmurs. The abdomen was soft and non-tender, with normal bowel sounds and no hepatosplenomegaly. The LSCS scar site was healthy. A complete neurological examination could not be performed because she was not fully oriented. However, reflexes in all limbs were normal with bilateral flexor plantar responses, and pupils were bilaterally normal and reactive. Signs of meningeal irritation were absent.
High-resolution computed tomography (HRCT) revealed ground-glass opacities in bilateral lung fields indicative of acute respiratory distress syndrome (ARDS). Ultrasound of the whole abdomen showed a bulky uterus with an echogenic collection in the uterine cavity.
The following blood parameters were tested after admission: hemoglobin 7.4 g/dL; total leucocyte count (TLC) 28,000/μL; platelet count 177,000/μL; C-reactive protein (CRP) 275 mg/dl; urea 25 mg/dl; creatinine 0.64 mg/dl; sodium 150 mEq/L; potassium 2.97 mEq/L; and procalcitonin (PCT) 0.816 ng/mL (reference normal <0.500 ng/mL).
Chest X-ray revealed bilateral heterogeneous opacities, raising the suspicion of ARDS or bronchopneumonia (Figure 1A). The patient was provisionally treated for hospital-acquired pneumonia with ARDS. Blood for culture and susceptibility testing was sent from the indwelling central line, and a new central line was inserted at another site. The patient was started on meropenem and vancomycin.
She was transferred to the ICU. Elective intubation was performed, and ventilator settings were adjusted according to ARDS status. Even after 72 hours of antibiotic treatment, no clinical improvement was noted. The TLC and CRP levels, which were measured serially, increased. The patient’s PCT level improved (0.364 ng/mL). After 48 hours, the provisional report of blood culture was sterile. Based on the clinical history and lack of improvement, empirical antifungal therapy with intravenous caspofungin was added. The meropenem and vancomycin were withheld, and polymyxin B with tigecycline was started, given persistent fever spikes and worsening general condition. This decision was also made due to the increasing prevalence of carbapenem-resistant Acinetobacter baumannii at our institute.
Final blood culture results showed that the Candida albicans strains were sensitive to caspofungin and voriconazole; hence, treatment with caspofungin was continued. There was no significant clinical improvement even after seven days of antibiotic or antifungal therapy. Therefore, blood and endotracheal tube aspirate were re-sent for culture and susceptibility testing, and antibiotics were changed to ceftazidime, teicoplanin, and doxycycline. The culture of endotracheal tube aspirate yielded no growth. The repeat blood culture grew Candida glabrata. Candidemia was further evaluated using contrast-enhanced computed tomography (CECT) of the thorax and abdomen. Imaging revealed bilateral lower lobe consolidations with multiple peribronchial parenchymal opacities, ground-glass opacities, interstitial thickening, and some cavitary changes, indicative of an atypical infection with ARDS.
Additionally, CECT revealed a defect in the anterior myometrial wall of the uterus with fistulous communication to the skin, likely a utero-cutaneous fistula secondary to LSCS wound dehiscence (Figure 1B).
Figure -1: Chest X-ray and CT abdomen of the patient during hospitalization. X-ray (A) at presentation showing bilateral heterogenous infiltrates s/o ARDS. CECT of the abdomen (B) shows a defect (white circle) in the anterior myometrial wall of the uterus with fistulous communication to the skin, likely a utero-cutaneous fistula.
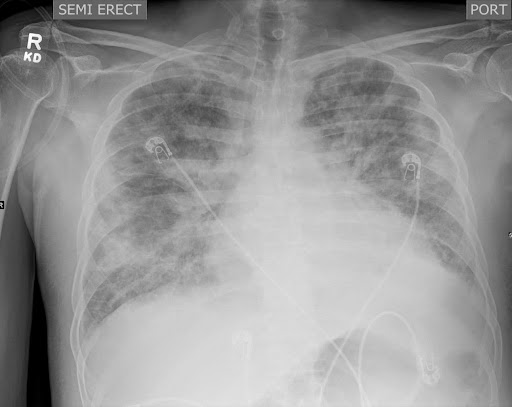
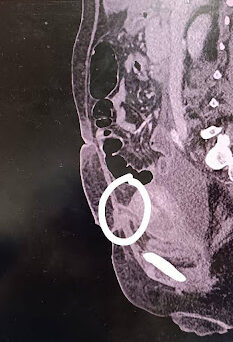
[A] [B]
Later, pus was observed oozing from the surgical site, but cultures from pus swabs were sterile. An Obstetrics and Gynecology consultation was sought, and high vaginal swabs for potassium hydroxide mount and cultures were obtained, both of which were negative. There was a suspicion of resistance to monotherapy, and intravenous voriconazole (200 mg BD) was added as a second antifungal agent. TLC and CRP were performed sequentially and showed a declining trend. Blood was obtained for culture after seven days of dual antifungal treatment and was sterile. Caspofungin was withdrawn after 15 days of therapy, and the patient was switched to oral fluconazole 800 mg. Given the patient’s current condition, surgical repair of the fistula was postponed.
The patient’s sensorium improved, her oxygen requirement decreased, she became afebrile, and her ventilator support was weaned off. Pulmonary rehabilitation was performed, and she was discharged and advised to continue treatment with oral fluconazole for two more weeks.
DISCUSSION
Persistent candidemia, defined as the continued presence of Candida species in the bloodstream for more than 72 hours despite appropriate treatment, poses a significant clinical challenge.11 This case highlights the complexities of managing such infections, particularly in postpartum patients.
In this case, the patient exhibited persistent candidemia, likely due to a útero-cutaneous fistula, an uncommon complication following a cesarean section. This condition often necessitates a multidisciplinary approach, including surgical intervention, source control, and tailored antifungal therapy. Imaging plays a critical role in identifying underlying sources of infection that may not be apparent clinically. In this case, imaging revealed the fistula, guiding the treatment strategy.
Managing candidemia typically involves using echinocandins, azoles, or amphotericin B formulations.11 While monotherapy is often effective, this case demonstrates the potential need for dual-antifungal therapy in refractory cases. The combination of caspofungin and voriconazole led to clinical improvement, suggesting that dual therapy may be beneficial in cases where single-agent therapy fails. Studies have shown mixed results regarding the efficacy of combination therapy (Table 1). Andes et al. found no significant difference in outcomes between monotherapy and combination therapy.12 However, Pappas et al. reported improved survival with dual-antifungal therapy in specific patient populations.7 In an in vitro study by Barchiesi et al., terbinafine plus fluconazole combination appeared synergistic.13 Rex et al. conducted a study in which a combination of amphotericin and fluconazole caused more rapid clinical improvement than monotherapy.14 In another study by Nivoix et al., the combination of caspofungin and amphotericin B was effective in 71% of the cases.15 In another analysis by Yang et al., the combination of caspofungin and voriconazole was not more effective than caspofungin alone.16 Further research is necessary to establish guidelines for dual therapy, particularly in patients with persistent candidemia and complex underlying conditions.
Table 1. Studies on dual antifungal therapy in candidiasis
|
S.no |
Author, year |
Study population/ study methodology |
Combined antifungal therapy |
Outcome |
|
1 |
Barchiesi et al 1997 (15) |
In vitro study |
Terbinafine + fluconazole |
Effective synergism was seen between the two drugs |
|
2 |
Rex et al., 2003 (16) |
Invasive candidiasis |
Fluconazole +amphoterecinB |
Combination therapy was better than the monotherapy group |
|
3 |
Nivoix et al, 2006(17) |
9 cases of candidiasis |
Caspofungin + amphotericin B/azole |
71% of patients responded to dual agents |
|
4 |
Yang et al, 2022 (18) |
Ten patients of proven candidiasis received combination therapy, 23 got caspofungin alone, and 4 got amphotericin B |
Caspofungin monotherapy vs amphotericin monotherapy vs. caspofungin + voriconazole |
The caspofungin group had better results (80.0%) than the caspofungin and voriconazole group (47.1%) |
This case also underscores the importance of considering less common sources of infection, such as útero-cutaneous fistulas, in patients with persistent candidemia. Surgical site infections, especially following obstetric procedures, can be a significant source of morbidity and require thorough investigation and management. The patient’s initial non-responsiveness to standard antifungal therapy highlighted the need for repeated blood cultures and comprehensive imaging to identify the infection source.
Additionally, the role of central lines in the development and persistence of candidemia cannot be overstated. Central line-associated bloodstream infections are a common cause of healthcare-associated infections and can complicate the clinical picture.17 In this case, despite a central line, the probable source attributed to the utero-cutaneous fistula emphasizes the need for a thorough and systematic approach to source identification.
CONCLUSIONS
This case demonstrates the challenges in managing persistent candidemia, particularly in the postpartum period. The successful use of dual-antifungal therapy in this patient highlights the potential benefits of combination treatment in refractory cases. Comprehensive imaging and a multidisciplinary approach are crucial in identifying and managing uncommon sources of infection. Further studies are needed to establish definitive guidelines for using dual-antifungal therapy and explore managing complex candidemia cases.
INFORMED CONSENT
Written informed consent was obtained from the patient. Confidentiality of the patient was maintained in the article.
CONFLICTS OF INTEREST STATEMENT
The authors declare no conflict of interest.
SOURCE OF FUNDING
None
AUTHOR’S CONTRIBUTION
JP: Conceptualization; Investigation; Supervision; Review & Editing
AB: Investigation; Writing the draft
TS: Supervision; Review & Editing
REFERENCES
-
Bassetti M, Righi E, Ansaldi F, et al. A multicenter study of septic shock due to candidemia: outcomes and predictors of mortality. Intensive Care Med. 2014;40(6):839-45.
Cornely OA, Bassetti M, Calandra T, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18 Suppl 7:19-37.
Briones-Claudett KH, Briones-Claudett HM, Murillo Vasconez RA, et al. An Unusual Case of Severe Pneumonia Caused Due Candida Tropicalis With a Favorable Clinical Response to Antifungals in a Nonimmunocompromised Patient From the Community. J Investig Med High Impact Case Rep. 2023;11:23247096231154652.
Sobel JD, Faro S, Force RW, et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol. 1998;178(2):203-11.
Yapar N. Epidemiology and risk factors for invasive candidiasis. Ther Clin Risk Manag. 2014;10:95-105.
Barantsevich N, Barantsevich E. Diagnosis and Treatment of Invasive Candidiasis. Antibiotics (Basel). 2022;11(6):718.
Pappas PG, Kauffman CA, Andes DR, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):e1-e50.
León C, Ruiz-Santana S, Saavedra P, et al. A bedside scoring system (“Candida score”) for early antifungal treatment in non-neutropenic critically ill patients with Candida colonization. Crit Care Med. 2006;34(3):730-7.
Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20(1):133-63.
Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(5):503-5.
Li WS, Chen YC, Kuo SF, Chen FJ, Lee CH. The Impact of Biofilm Formation on the Persistence of Candidemia. Front Microbiol. 2018;9:1196.
Andes DR, Safdar N, Baddley JW, et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis. 2012;54(8):1110-22.
Barchiesi F, Falconi Di Francesco L, Scalise G. In vitro activities of terbinafine in combination with fluconazole and itraconazole against isolates of Candida albicans with reduced susceptibility to azoles. Antimicrob Agents Chemother. 1997;41(8):1812-4.
Rex JH, Pappas PG, Karchmer AW, et al. A randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in non-neutropenic subjects. Clin Infect Dis. 2003;36(10):1221-8.
Nivoix Y, Zamfir A, Lutun P, et al. Combination of caspofungin and an azole or an amphotericin B formulation in invasive fungal infections. J Infect. 2006;52(1):67-74.
Yang Q, Xie J, Cai Y, et al. Efficacy and Safety of Combination Antifungals as Empirical, Preemptive, and Targeted Therapies for Invasive Fungal Infections in Intensive-Care Units. Infect Drug Resist. 2022;15:5331-44.
Ostrosky-Zeichner L, Sable C, Sobel J, et al. Multicenter retrospective development and validation of a clinical prediction rule for nosocomial invasive candidiasis in the intensive care setting. Eur J Clin Microbiol Infect Dis. 2007;26(4):271-6.
Submit a Manuscript:
Copyright © Author(s) 2024. JASPI- Journal of Antimicrobial Stewardship Practices and Infectious Diseases.

