Surveillance of Antimicrobial Consumption in India: Relevance and the Road Ahead
INTRODUCTION
Inappropriate use of antimicrobials is among the major drivers for the development of antimicrobial resistance (AMR).1 Therefore, there is an urgent need to understand the burden of inappropriate antimicrobial use, without the knowledge of which it is impossible to develop the corrective and preventive strategies.
In order to estimate the extent of inappropriate antimicrobial use, it is imperative to closely observe, accurately document and diligently analyse all aspects of antimicrobial use (AMU), which in other words explains the need for antimicrobial surveillance which is to collect data related to antimicrobial use. It begins with quantification of antimicrobial consumption (AMC) followed by exploration of factors contributing to antimicrobial use (AMU).
GLOBAL ACTION PLAN
To preserve the effectiveness of antimicrobials and ensure equitable access WHO adopted Global Action Plan in 2015. Its five objectives include raising awareness, strengthening surveillance, reducing infection incidence, optimizing antimicrobial use, and ensuring sustainable investment. Optimizing use is central, supported by antimicrobial stewardship programmes. In 2017, WHO revised its Model List of Essential Medicines, introducing the AWaRe classification (Access, Watch, Reserve) to guide rational prescribing, improve treatment outcomes, and preserve last-line drugs. Complementary measures include strengthening diagnostics, building laboratory capacity, and establishing reliable supply chain systems.1
The first and foremost challenge for implementation of GAP was to understand the global burden of AMR. It was necessary so as to convince the healthcare organisations the need to adopt infection prevention and control measures. Recognizing this as an urgent need, WHO initially initiated standardized surveillance of resistant pathogens, through Global Antimicrobial Resistance Surveillance System (GLASS) in 2015. As predicted, In the first year, high ratio of resistance was observed among tested isolates. This observation raised the necessity to understand factors that contribute to AMR. Therefore, a new module on surveillance of antimicrobial use, and consumption was added. GLASS standardizes global methods, enabling trustworthy comparisons and strengthening One Health strategies 1 In response to the lack of antimicrobial consumption (AMC) data, particularly in low- and middle-income countries, WHO also initiated the global programme on surveillance of antimicrobial consumption. In 2016, it developed WHO global methodology and began data collection for 2014–2016 across selected countries.[1] In response to the call, 36 countries enrolled and reported antimicrobial use (AMU) in 2016 which gradually increased to 63 in 2022.2
ANTIMICROBIAL CONSUMPTION METRICS
AMC and AMU are two approaches to monitoring antimicrobial surveillance. Both approaches serve distinct purposes and complement rather than replace each other. 3
In its third global report, GLASS – Antibiotic use data for AMU, WHO introduced the term “medicine-level” AMU data (m-AMU) data for Antimicrobial Consumption (AMC) which includes data that provides estimates of volume of medicine used without any other context and the term “clinical-level” AMU data (c-AMU) for any data in which information of antimicrobial used is associated with clinical information.4
At global level, as the only quality indicator endorsed by member states is proportion of total use of Access group antimicrobials, with the 70% global target by 2030, based on m-AMU or AMC, 4 WHO developed a standardized protocol for collecting and reporting national AMC data to WHO GLASS. This includes information on proprietary and generic names, active substances, routes of administration, unit strengths, packaging, and the number of units consumed. The WHO methodology applies the Anatomical Therapeutic Chemical classification system to categorize antimicrobials. 3 The different metrics for AMC are presented in Table No.1.
Table-1: Antimicrobial Consumption metrics 5, 6, 7
Indicator | Definition / Application | Interpretation Example | Usefulness |
DDD per 1000 inhabitants per day (DID) | Number of Defined Daily Doses (DDDs) utilized per 1000 people each day | 10 DDDs/1000/day → 1% of the population receives the drug daily | Useful for drugs used chronically; assumes PDD ≈ DDD |
DDD per 100 bed days | Number of DDDs used per 100 inpatient bed days | 70 DDDs/100 bed days of hypnotics → 70% of inpatients receive 1 DDD daily | Useful for in-hospital drug use |
DDD per patient | DDDs consumed per patient during a study period | If PDD = DDD, equals number of treatment days per patient | Indicates treatment intensity/exposure |
DDDs per inhabitant per year | Average DDDs used per inhabitant annually | 5 DDDs/inhabitant/year → every inhabitant receives a 5-day course yearly | Useful for short-term drug use |
Days of Therapy (DOT) | DOT (Aggregate sum of days for which any amount of a specific antimicrobial agent was administered to individual patients) | 50 DOT → 50 individual antimicrobial drug days. This measures antimicrobial density | Useful for comparison antimicrobial drug burden of facilities/hospitals especially for paediatric units |
Length of Therapy (LOT) | The number of days that a patient receives systemic antimicrobial agents, irrespective of the number of different antibiotics. | 50 LOT/1000 PDs→ 50 days of any systemic antimicrobial use This measures total length of any systemic antimicrobial therapy | Useful to evaluate durations (in days) of antimicrobial therapy |
Standardized Antimicrobial Administration Ratio (SAAR) | Risk-adjusted measure calculated by dividing the observed antimicrobial use by the predicted antimicrobial use (calculated using predictive models). | SAAR = 1means observed antimicrobial use is equal to predicted antimicrobial use A target of 0.95 is optimal | Useful to analyze impact of interventions. |
Abbreviations: PDD-Prescribed Daily Dose
To quantify AMC, the metric of defined daily doses (DDD) is mostly used, representing the assumed average maintenance dose per day of a drug for its main indication in adults. To account for population size, antimicrobial consumption is commonly expressed as DDDs per 1000 inhabitants per day (DID), which can be interpreted as the proportion of individuals on antibiotic treatment at any given time. 3
In addition, AMC can be reported as total DDDs by ATC subgroup, total weight in tonnes, relative proportions by route of administration and AWaRe category, or the DU75% index identifying antibiotics that constitute 75% of total consumption. 3
According to GLASS report – Antibiotic use data for 2022, precisely m-AMU data was reported by the 60 member states. According to this report, the median total antibiotic consumption was 18.3 DID, which indicates that 18.3% of patients were receiving antimicrobials daily. Antimicrobial use was observed to be highest in the South-East Asia with 26.6 DID and lowest in western pacific region. Globally, only 58% of the member states achieved the 60% Access target and only 31.7% achieved 70% Access target. Furthermore, it also reported that member states with higher levels of AMC utilized fewer Access and more Watch antibiotics. Extended-spectrum penicillins (17.9%) followed by macrolides (14.7%) were among the most prescribed antimicrobials.4
NATIONAL ACTION PLAN
Cholera, caused by Vibrio cholerae, is characterized by rapid onset of profuse watery diarrhea and can lead to severe dehydration. In an outbreak setting, the direct microscopic examination of stool samples can reveal the classic darting motility of V. cholerae, i.e. “shooting star” motility. This rapid motility is a unique diagnostic feature that can be observed under a wet mount preparation, providing immediate clues for diagnosis. Such rapid identification is crucial for outbreak control, allowing for the swift administration of antibiotics and rehydration therapy, preventing further spread and saving lives. Similarly, the diagnostic clues in Albert’s stain coupled with the clinical presentation, allow rapid identification of Corynebacterium diphtheriae, enabling prompt initiation of diphtheria antitoxin and appropriate antibiotic therapy. This case exemplifies the pivotal role of traditional microbiological techniques, such as staining, in identifying pathogens in a timely manner during outbreaks.
In addition, NCDC initiated National Antimicrobial Consumption Network (NAC-Net) in 2021 across 30 hospitals and expanded to 36 tertiary care hospitals by 2024. Through this network, NCDC is capturing antimicrobial consumption data using ATC/DDD methodology and antimicrobial utilization as per AWaRe classification. 9
Figure-1: NAP-AMR Plan with Focus on Strategic Priority-4: Optimise Use of Antimicrobial Agents
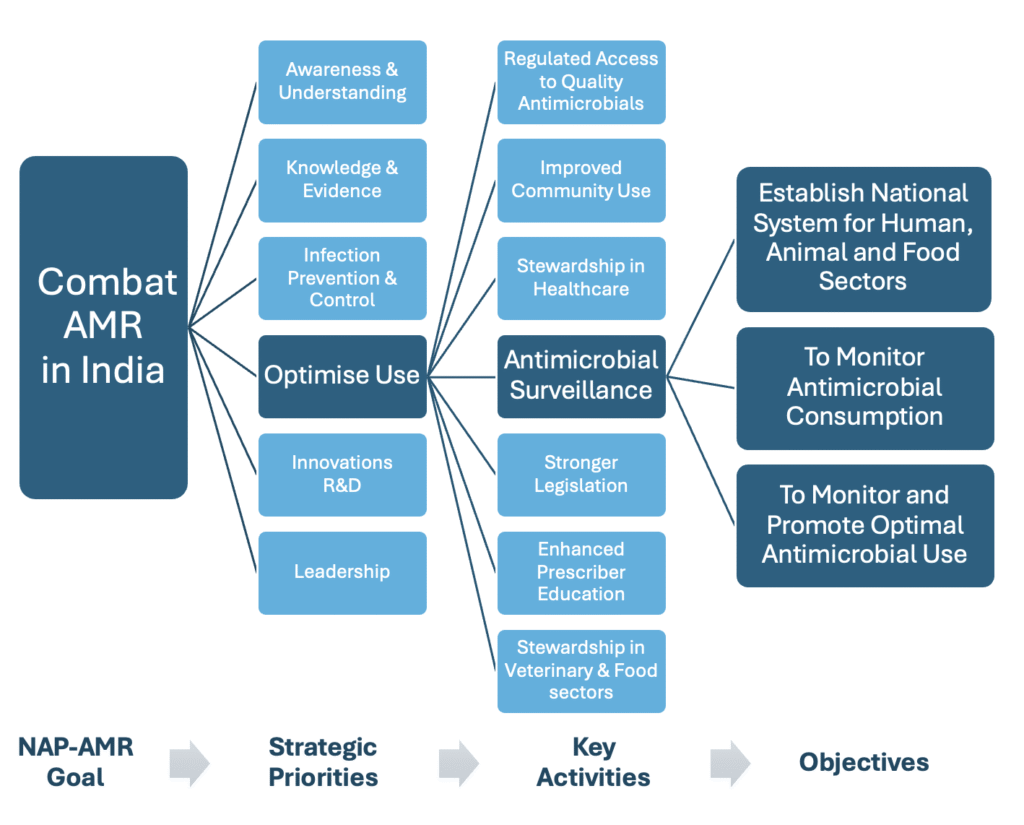
ICMR initiated a nationwide Antimicrobial Stewardship Programme (AMSP), developed AMSP guidelines and rolled out stewardship programs in regional centres. The program’s objectives included monitoring antibiotic consumption in ICUs using the ICMR tool (DOTS/DDD) and developing institution-specific antimicroobial policies in Phase I in 2019. 10 In Phase II, ICMR designed an electronic case record form to capture antimicrobial surveillance data for consumption and use, which is being implemented across hospitals with its support for three years in 2022. The project is ongoing and data is yet to be released on antimicrobial consumption which shall help in understanding utilization patterns across India.
India initially enrolled in GLASS-AMR in 2017, focusing on resistance patterns.11 With continued consistent efforts of both NCDC and ICMR, India achieved another milestone by enrolling in GLASS-AMU in 2024. 12 This aligns with a key objective of the National Action Plan on AMR (NAP-AMR)—to monitor AMC nationally. 8
ANTIMICROBIAL CONSUMPTION DATA REPORTS
NCDC coordinated National Antimicrobial Consumption Network (NAC-NET) study13 in 2021, Koya et al., data on private antibiotic consumption in India using PharmaTrac in 201914 and ASPIRE-II SASPI point prevalence study in 202315 report that antimicrobial utilization was highest from Watch group (54.8% – 57.3%) and third generation cephalosporins as the most prescribed, which emphasize the high proportion of broad-spectrum antimicrobial use. Furthermore, NAC-NET reported double coverage of gram-negative infections in 25%, while, ASPIRE II study group reported double anaerobic coverage which call for broader policy implementation to optimize antimicrobial use across healthcare institutions in India.
ROAD AHEAD
Perhaps, addition of GLASS- AMU module triggered surveillance of antimicrobial consumption in member states of WHO. However, the data that is contributed still represents only the “tip of the iceberg.”
Recognizing this current status, NAP-AMR 2.0 is taking steps to reinforce the modalities and mechanisms for national antimicrobial surveillance. Henceforth, NCDC shall create awareness, impart training and involve tertiary care healthcare organizations at national and state level and ICMR shall train secondary level healthcare organizations by development of antimicrobial stewardship program (AMSP) guidelines for healthcare set ups at all levels and conduct training programs for implementation of the AMSP. 9
In addition, NAP-AMR 2.0 calls for antimicrobial surveillance in veterinary settings, animal husbandry, fisheries, agricultural farms & food processing units. 9 India is thus embarking on a journey to optimize antimicrobial use at all levels across all settings. The vision shall become reality only when all healthcare professionals across all disciplines understand the importance of antimicrobial surveillance and willingly share the antimicrobial consumption data so as to enhance India’s contribution to global and national AMR containment efforts.
CONCLUSION
Surveillance of antimicrobial consumption and use is crucial to combat the escalating threat of AMR. To strengthen national and global efforts under the NAP, it is imperative to encourage and engage more organizations to actively contribute and share their data. Only through collective participation can we ensure a comprehensive understanding of antimicrobial practices.
ACKNOWLEDGEMENT:
None
CONFLICT OF INTEREST STATEMENT:
Authors declare no conflict of interest.
SOURCE OF FUNDING:
None
DECLARATION FOR THE USE OF GENERATIVE ARTIFICIAL INTELLIGENCE (AI) IN SCIENTIFIC WRITING: Utilized ChatGPT only for refining language. It has not been used either to generate the content or to critically analyse any piece of information/data.
REFERENCES
1. WHO report on surveillance of antibiotic consumption: 2016-2018 early implementation. Geneva: World Health Organization; 2018.
2. World health Organization. Glass AMU data. (2024). Accessed: 2025: https://worldhealthorg.shinyapps.io/glass-dashboard/_w_b666ad6713df49a2b89ec956f2c05243/#!/amu
3. GLASS manual on the management of antimicrobial consumption data. Geneva: World Health Organization; 2020.
4. Global Antimicrobial Resistance and Use Surveillance System (GLASS) report. Antibiotic use data for 2022. Geneva: World Health Organization; 2025.
5. Norwegian Institute of Public Health WHO Collaborating Centre for Drug Statistics Methodology. Use of ATC/DDD. (2025). Accessed: 2025: https://atcddd.fhi.no/use_of_atc_ddd/#indica
6. Antimicrobial Use and Resistance (AUR) Module: National Health Safety Network, CDC; Accessed: 2025: https://www.cdc.gov/nhsn/pdfs/ps-analysis-resources/aur/au-qrg-saartables.pdf
7. Benic MS, Milanic R, Monnier AA, Gyssens IC, Adriaenssens N, Versporten A et al., the DRIVE-AB WP1 group , Metrics for quantifying antibiotic use in the hospital setting: results from a systematic review and international multidisciplinary consensus procedure, Journal of Antimicrobial Chemotherapy. 2018:73(6): vi50–vi58
8. National Action Plan on Antimicrobial Resistance (NAP-AMR) 2017-2021.Ministry of Health and Family Welfare, Government of India.(2017). Accessed: 2025: https://ncdc.mohfw.gov.in/wp-content/uploads/2024/03/File645.pdf
9. National Action Plan on Antimicrobial Resistance (NAP-AMR) (2025-2029) One Health Approach. Version 2.0: Government of India; 2025. Accessed: 2025: https://ncdc.mohfw.gov.in/wp-content/uploads/2025/11/National-Action-Plan-on-Antimicrobial-Resistance-2.0.pdf
10. ICMR. India’s Antimicrobial Resistance Surveillance and Research Initiative. AMSP Generic Protocol. Accessed:2025: https://iamrsn.icmr.org.in/index.php/resources/amsp-protocol
11. WHO. Country, territory or area profiles. (2023). Accessed: 2025: https://worldhealthorg.shinyapps.io/glass-dashboard/_w_3f77c98ec6ef4903a1dba6c8eb03ccf3/#!/cta-profiles
12. WHO. GLASS Enrolment Map October 2024. Accessed: 2025: https://cdn.who.int/media/images/default-source/headquarters/teams/antimicrobial-resistance-division-(amr)/surveillance-prevention-and-control-(spc)/control-and-response-strategies-(csr)/map-participation.png?sfvrsn=640c79a_1
13. National Programme on AMR Containment. National centre for Disease Control (NCDC), Directorate General of Health Services. Report of the First Multicentric Point Prevalence Survey of Antibiotic Use at 20 NAC-NET sites, India. 2021. Accessed: 2025: https://ncdc.mohfw.gov.in/wp-content/uploads/2024/03/FinalNACNETReport.pdf
14. Koya SF, Ganesh S, Selvaraj S, Wirtz JV, Galea S, and Rockers PC. Consumption of systemic antibiotics in India in 2019. The Lancet Regional Health – Southeast Asia. 2022;4: 100025
15. Bhattacharjee S, Mothsara C, Shafiq N, Panda PK, Rohilla R, Kaore SN et al., ASPIRE II study group (SASPI). Antimicrobial prescription patterns in tertiary care centres in India: a multicentric point prevalence survey. EClinicalMedicine. 2025;82:103175.
Submit a Manuscript:
Copyright © Author(s) 2025. JASPI- Journal of Antimicrobial Stewardship Practices and Infectious Diseases.

