Metronidazole Refractory Giardiasis Complicating the Management of Rhabdomyosarcoma in a Child
JASPI March 2024/ Volume 2/Issue 1
Copyright: © Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Khan S, Kharchandy H L, Kataria B.Metronidazole Refractory Giardiasis Complicating the Management of Rhabdomyosarcoma in a Child. JASPI.2024;2(1):15-19 DOI:10.62541/jaspi017
ABSTRACT
Giardiasis caused by Giardia duodenalis is one of the leading causes of gastroenteritis, especially in developing countries. The scenario may be further complicated by emerging metronidazole refractory cases worldwide. We present a case of a 2-year-old girl on chemotherapy for metastatic para meningeal rhabdomyosarcoma. She had a 15 day history of persistent diarrhea of foul-smelling loose stools, without fever. Light microscopy of stool wet mounts revealed cysts and trophozoites of Giardia duodenalis. The patient was managed with nitazoxanide after metronidazole proved ineffective clinically. This case highlights the importance of timely diagnosis and management of infectious diseases and rising antimicrobial resistance among parasites.
KEYWORDS: Antimicrobial resistance; diarrhea; Giardia duodenalis; metronidazole refractory; nitazoxanide
INTRODUCTION
Giardiasis is an enteric infection caused by the protozoan parasite Giardia duodenalis. It is one of the leading causes of treatable infectious gastroenteritis globally. The prevalence of Giardia infections is reported to be higher in low-income countries than in high-income countries.1 Most of the disease burden is from developing countries, influenced by climate, poverty, and lack of access to health services. Hence, the World Health Organisation included Giardiasis in the ‘Neglected Diseases Initiative’ in September 2004.2 Giardia infection generally leads to subacute diarrhea that begins 1 to 2 weeks after consumption of food or water contaminated with cysts of Giardia duodenalis.3 The patients may develop subclinical infections or present with a wide range of symptoms, including profuse, fatty diarrhea, abdominal cramps, nausea, wasting, dehydration, and growth stunting in children.
Fever and other systemic symptoms are typically absent.3-5 Various risk factors for acquiring this infection include consumption of unsafe food or water, contact with Giardiasis patients or carriers, especially in childcare settings, travel to endemic regions, oral-anal sexual contact, poor sanitation, and overcrowding.6
Most laboratories diagnose giardiasis by microscopic identification of Giardia’s cysts (and less commonly trophozoites) in stool specimens. Testing at least three stool samples over a week apart is recommended due to variable shedding.1 Antiparasitic drugs are available for treating Giardia infections, like metronidazole, tinidazole, nitazoxanide, paromomycin, quinacrine, and furazolidone.7 Nitroimidazoles are considered first-line treatment, and a 5 to 7-day metronidazole can cure over 90% of individuals.8 However, metronidazole-refractory giardiasis is being increasingly reported.9 Giardia duodenalis, along with other parasites are considered opportunistic parasite in patients with immunosuppressive conditions like HIV, cancer, cancer chemotherapy, prolonged steroid therapy.10
We report a case of metronidazole refractory giardiasis in a young girl on chemotherapy for metastatic para meningeal rhabdomyosarcoma.
CASE REPORT
A 2-year-old girl was admitted to the medical oncology ward of our tertiary care cancer hospital in June 2023 with complaints of persistent diarrhea for over 15 days. There were 4-5 episodes of foul-smelling loose stools, not associated with fever. The patient was admitted for detailed evaluation, as she did not improve on outpatient-based treatment.
The patient was a known case of metastatic para meningeal rhabdomyosarcoma (IRSG group-IV), immunopositive for desmin and myogenin but negative for NKX2, MIC2, and synaptophysin. She was diagnosed at our hospital following left lateral rhinotomy at a private hospital and had completed 11 cycles of VAC (vincristine 0.5 mg IV, actinomycin-D 0.375 mg IV, cyclophosphamide 500 mg in 500 mL 5% dextrose) chemotherapy.
The patient had a history of multiple hospital admissions for chemotherapy sessions and various chemotherapy-related complications like chemotherapy-induced nausea and vomiting (CINV) and febrile neutropenia, which were effectively managed. There was a history of admission three months back for fever and loose stools, wherein the patient improved with IV antibiotics (cefoperazone-sulbactam, amikacin, and metronidazole) and supportive care.
During the present admission, the general and systemic examinations of the child were within normal limits, with a soft and non-tender abdomen and no signs of dehydration. No significant abnormality was detected on ultrasonography of the abdomen and pelvis. Her complete hemogram revealed anemia (9.6g/dL). Liver function tests showed slightly raised serum glutamic oxaloacetic transaminase (64.9 U/L) and alkaline phosphatase (134 IU), but renal function tests were within the normal range. The patient was non-reactive for Hepatitis-B, Hepatitis-C, and Human immunodeficiency virus.
A freshly passed stool specimen was received in the Microbiology laboratory on June 13, 2023. On gross examination, the specimen was yellowish and loose in consistency; mucous was present, while blood was absent. No parasitic elements were visible to the naked eye. Light microscopy of stool wet mounts prepared in normal saline and iodine revealed multiple oval double-walled cysts (measuring 10-14 µm) and pear-shaped trophozoites (measuring 10-20 µm) of Giardia duodenalis without red blood cells and pus cells (Figure 1A-B). Trophozoites showed falling leaf type motility in saline mount. The stool specimen was also positive for Giardia antigen when tested with a rapid immuno-chromatographic test (Vitassay, Huesca, Spain) that can simultaneously detect G. duodenalis, Cryptosporidium parvum, and E. histolytica (Figure 1C).
Figure 1. Microbiological confirmatory tests of the patient. Stool wet mount examination depicting cyst (A) and trophozoite (B) of Giardia duodenalis; The immunochromatographic test showed only the green control bands (small black arrow) and positive red band (big black arrow) for Giardia duodenalis (C).
[A] [B]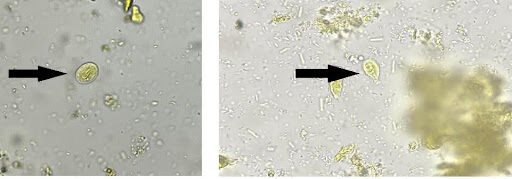
[C]
DISCUSSION
Giardiasis is also known as ‘beaver fever’ (although fever is a rare finding),6 because beavers serve as essential reservoirs, and water contamination with their feces can lead to Giardia outbreak.11,12 Most of the disease burden is from the developing world,1 with children at a relatively higher risk than adults due to poorly developed immune systems.13 The prevalence of G. duodenalis infection among Indian children is estimated to be around 10 to 20%.14 Cancer and its treatment lower patients’ immunity, which is a significant risk factor for giardiasis. Giardia is one of the most predominant parasites causing gastrointestinal infection among cancer patients.15-17 In the present case, the patient was under cancer chemotherapy for metastatic para meningeal rhabdomyosarcoma.
Factors like the non-specific nature of symptoms, a large proportion of subclinical diseases, the intermittent pattern of shedding of cysts, etc., can make the diagnosis difficult.6 In the present case, the diagnosis was made early by stool microscopy and antigen detection (rapid immunochromatography test) in stool specimens. Immunochromatography kits for antigen detection show low sensitivity but can be helpful in supportive diagnosis.18
Metronidazole (nitroimidazole) is the treatment of choice for 5 to 10 days.3 However, nitroimidazole resistance is increasingly being observed, and treatment failure may occur in up to 20% of cases.9,19 A study by Nabarro et al. suggests a rapid increase in nitroimidazole treatment failure in London, from 15.1% in 2008 to 20.6% in 2011 and 40.2% in 2013.20 Out of 456 cases in a recent treatment ladder study, only 54% (248/456) were cured by metronidazole.21 Reduced activity of nitroimidazoles could be due to multiple mechanisms, including reduced drug uptake, downregulation of pyruvate ferredoxin oxidoreductase (PFOR) pathway, downregulation of nitroreductase-1, upregulation of nitroreductase-2, etc.9 Assemblage B Giardia isolates are associated with higher refractory rates, as demonstrated by Yadav et al. in their study, wherein all eight refractory Giardia isolates belonged to assemblage B.22 Various methods are available for in-vitro susceptibility testing for Giardia, namely subculture in liquid medium (SCLM), tritiated thymidine uptake (3H-TdR), MTT reduction, Fluorogenic dye staining, Cell morphology, automated image-based assays, calorimetric assays, ATP content measurement, etc. These methods detect the viability of drug-exposed giardia trophozoites and could assist in the management of treatment failure and for newer drug evaluation.23-26
The present case did not respond to metronidazole clinically and was ultimately treated with nitazoxanide. Some investigators have tried the management of metronidazole refractory giardiasis with tinidazole, secnidazole, nitazoxanide, paromomycin, neomycin, furazolidone, quinacrine, albendazole, mebendazole, etc. as monotherapy or in combination. Combination drugs generally perform better than monotherapy. Metronidazole, combined with albendazole, has shown a cure rate of up to 90%, whereas quinacrine has an 81% to 100% cure rate. Quinacrine, nitazoxanide, or a combination of a benzimidazole, such as albendazole or mebendazole, and a nitroimidazole are considered the most effective second-line regimen.22,27-28
CONCLUSION
Giardiasis is preventable and treatable, but its diagnosis may be missed due to the non-specific nature of symptoms and the high rate of subclinical infections. As in this case, the symptoms could be confused with the adverse effects of antibiotics and anti-cancer drugs. Drug resistance is also emerging in protozoans, just like bacteria and fungi, should be suspected clinically if not responding to first line therapy. Therefore, timely suspecting, diagnosis, and treatment of such infections are necessary to prevent complications.
INFORMED CONSENT
The patient’s guardian provided consent to publish the case report. Confidentiality of the patient was maintained in the article.
CONFLICT OF INTERESTS STATEMENT
The authors declare no conflict of interest.
SOURCE OF FUNDING
None
AUTHORS’ CONTRIBUTIONS
SK: Conceptualization; Data curation; Analysis; Writing the draft
HLK: Review and editing.
BK: Supervision, Validation, Review & editing
REFERENCES
Minetti C, Chalmers RM, Beeching NJ, Probert C, Lamden K. Giardiasis. BMJ. 2016;355:i5369.
Savioli L, Smith H, Thompson A. Giardia and Cryptosporidium join the ‘Neglected Diseases Initiative’. Trends Parasitol. 2006;22(5):203-8.
Adam RD. Giardia duodenalis: Biology and Pathogenesis. Clin Microbiol Rev. 2021;34(4):e0002419.
Centers for Disease Control and Prevention (CDC). Parasites-Giardia. Pathogen and Environment. Accessed December 22, 2023. https://www.cdc.gov/parasites/giardia/pathogen.html
Rojas-López L, Marques RC, Svärd SG. Giardia duodenalis. Trends Parasitol. 2022;38(7):605-6.
Centers for Disease Control and Prevention (CDC). Parasites-Giardia. General Information. Accessed December 22, 2023. https://www.cdc.gov/parasites/giardia/general-info.html
Centers for Disease Control and Prevention (CDC). Parasites-Giardia. Diagnosis and Treatment Information for Medical Professionals. Accessed December 22, 2023. https://www.cdc.gov/parasites/giardia/medical-professionals.html
Gardner TB, Hill DR. Treatment of giardiasis. Clin Microbiol Rev. 2001;14(1):114-28.
Carter ER, Nabarro LE, Hedley L, Chiodini PL. Nitroimidazole-refractory giardiasis: a growing problem requiring rational solutions. Clin Microbiol Infect. 2018;24(1):37-42.
Gupta RK, Gupta P. Opportunistic Parasitic Infections. In: Gupta RK, Gupta P, eds. Pathology of Opportunistic Infections [Internet]. Singapore: Springer Singapore; 2017:131–45. http://link.springer.com/10.1007/978-981-10-1669-1_5
Monzingo DL, Hibler CP. Prevalence of Giardia spp. in a Beaver colony and the resulting environmental contamination. J Wildl Dis. 1987;23(4):576–85.
Encyclopedia. Giardiasis. Accessed December 22, 2023. https://www.encyclopedia.com/medicine/diseases-and-conditions/pathology/giardiasis
Shrimali T, Srivastava S, Mohammad N, John N, Tak V, Saxena R. Giardiasis in an Infant With Fibrosarcoma: A Case Report. Infect Disord Drug Targets. 2023;23(7):82-5.
Kalavani S, Matin S, Rahmanian V, Meshkin A, Taghipour A, Abdoli A. Prevalence of Giardia duodenalis among Asian children: a systematic review and meta-analysis. Int Health. 2024;16(2):133-43.
Mahdavi F, Sadrebazzaz A, Chahardehi AM, et al. Global epidemiology of Giardia duodenalis infection in cancer patients: a systematic review and meta-analysis. Int Health. 2022;14(1):5-17.
Esteghamati A, Khanaliha K, Bokharaei-Salim F, Sayyahfar S, Ghaderipour M. Prevalence of Intestinal Parasitic Infection in Cancer, Organ Transplant and Primary Immunodeficiency Patients in Tehran, Iran. Asian Pac J Cancer Prev. 2019;20(2):495-501.
Chen HH, Deng Y, Li Z, et al. [Prevalence and risk factors of Giardia lamblia infections among colorectal cancer patients in Henan Province]. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2022;34(4):370-7.
Khan S, Das A, Nandal M, Vashisth D, Vidyarthi AJ, Mirdha BR. Evaluation of a rapid immunochromatographic test for the diagnosis of intestinal protozoan infections among patients attending a rural outreach outpatient department in Northern India. Trop Doct. Published online December 21, 2023.
Muñoz Gutiérrez J, Aldasoro E, Requena A, et al. Refractory giardiasis in Spanish travellers. Travel Med Infect Dis. 2013;11(2):126-9.
Nabarro LE, Lever RA, Armstrong M, Chiodini PL. Increased incidence of nitroimidazole-refractory giardiasis at the Hospital for Tropical Diseases, London: 2008-2013. Clin Microbiol Infect. 2015;21(8):791-6.
Cañete R, Noda AL, Rodríguez M, et al. 5-Nitroimidazole refractory giardiasis is common in Matanzas, Cuba and effectively treated by secnidazole plus high-dose mebendazole or quinacrine: a prospective observational cohort study. Clin Microbiol Infect. 2020;26(8):1092.e1-1092.e6.
Yadav P, Tak V, Mirdha BR, Makharia GK. Refractory giardiasis: a molecular appraisal from a tertiary care centre in India. Indian J Med Microbiol. 2014;32(4):378-82.
Argüello-García R, Cruz-Soto M, Romero-Montoya L, Ortega-Pierres G. Variability and variation in drug susceptibility among Giardia duodenalis isolates and clones exposed to 5-nitroimidazoles and benzimidazoles in vitro. J Antimicrob Chemother. 2004;54(4):711-21.
Gut J, Ang KK, Legac J, Arkin MR, Rosenthal PJ, McKerrow JH. An image-based assay for high throughput screening of Giardia lamblia. J Microbiol Methods. 2011;84(3):398-405.
Hounkong K, Sawangjaroen N, Phongpaichit S. A colorimetric method for the evaluation of anti-giardial drugs in vitro. Exp Parasitol. 2011;127(2):600-3.
Chen CZ, Kulakova L, Southall N, et al. High-throughput Giardia lamblia viability assay using bioluminescent ATP content measurements. Antimicrob Agents Chemother. 2011;55(2):667-75.
Lalle M, Hanevik K. Treatment-refractory giardiasis: challenges and solutions. Infect Drug Resist. 2018;11:1921-33.
Bourque DL, Neumayr A, Libman M, Chen LH. Treatment strategies for nitroimidazole-refractory giardiasis: a systematic review. J Travel Med. 2022;29(1):taab120.
Submit a Manuscript:
Copyright © Author(s) 2024. JASPI- Journal of Antimicrobial Stewardship Practices and Infectious Diseases.

