Candidemia Due to Candida Kefyr in A Patient with Severe Acute Pancreatitis – An Identification after Death
JASPI September 2025 / Volume 3 /Issue 3
Copyright: © Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Subramanian J, Sikdar S, Priyadarshi M, et al.Candidemia due to Candida kefyr in a patient with severe acute pancreatitis – An identification after death. JASPI. 2025;3(3):37-40 DOI: 10.62541/jaspi102
ABSTRACT
Severe acute pancreatitis is frequently complicated by secondary infections that worsen outcomes, but fungal bloodstream infections are uncommon. We report a fatal case of Candida kefyr fungemia in a 38-year-old male with severe acute alcoholic pancreatitis. The patient presented with abdominal pain, vomiting, and altered sensorium, and developed multiorgan failure requiring intensive care, vasopressors, and dialysis. Despite broad-spectrum antibiotics and empiric caspofungin therapy, his condition deteriorated, and he succumbed. Post-mortem fungal cultures grew Candida kefyr, which was susceptible to fluconazole, voriconazole, amphotericin B, and caspofungin. Candida kefyr, previously considered a rare yeast, is now emerging as a pathogen particularly in critically ill and immunocompromised hosts. It demonstrates variable susceptibility patterns and has been associated with rapid acquisition of antifungal resistance, complicating management. This case shows the need of suspecting invasive fungal infection early in patients with severe pancreatitis and multi-organ failure who fail to respond to antibiotics. Timely identification and susceptibility-guided antifungal therapy remain critical for improving outcomes. Heightened clinical vigilance and improved diagnostic tools are essential for prompt recognition of C. kefyr infections in high-risk patients.
KEYWORDS: Candidemia, Candida kefyr, Severe acute pancreatitis
INTRODUCTION
Acute pancreatitis is a life-threatening condition with devastatingly high mortality. Severe acute pancreatitis is characterised by multiple organ failure along with systemic inflammatory response syndrome making them have high mortality. Infective complications are seen in most of these patients further increasing the risk of mortality. These infections can be either localised, confined to the necrotic pancreatic tissue or systemic in nature. Both bacterial and fungal infections
have been reported in such cases. Candida infections have been extensively documented in these patients in the form of intra-abdominal abscesses as well as candidemia1.
Most of the candida infections are due to C.albicans and C.tropicalis. Candida kefyr is one of the candida species that is quite rare in prevalence2. It has been reported as one of the emerging species of candida causing infections in the immunocompromised patients. It has been noted to have a varied susceptibility for the antifungal agents, especially amphotericin B and echinocandins. The biggest challenge in managing these infections is the accurate identification of such infection and initiating the appropriate treatment.
We present an interesting and rare case of Candida kefyr bloodstream infection in a patient with severe acute pancreatitis.
CASE PRESENTATION
A 38-year-old male, chronic alcoholic, with a recent history of binge intake was admitted with complaints of severe abdominal pain and multiple episodes of vomiting. He also had complaints of melena and altered sensorium. He was icteric, disoriented with right subconjunctival haemorrhage with diffuse abdominal tenderness. Ultrasound of the abdomen revealed bulky hypoechoic pancreas with peri pancreatic, mild ascites with bilateral pleural effusion. Amylase and lipase were elevated. Thrombocytopenia and leucocytosis were present (Table 1).
Parameter | Normal Range (Conventional Units) | D1 | D4 | D5 | D6 |
Hemoglobin (g/dL) | 12–16 | 8.3 | 8.4 | 7.8 | 8.2 |
Hematocrit / PCV (%) | 36–46 | 25.2 | 26.6 | 26.7 | 25.9 |
Total Leukocyte Count (/µL) | 4,000–11,000 | 8.07 | 20.79 | 20.68 | 16.8 |
Platelet Count (/µL) | 150,000–400,000 | 27 | 87 | 105 | 148 |
Urea (mg/dL) | 15–45 | 110 | 109 | 213 | 255 |
Creatinine (mg/dL) | 0.6–1.2 | 6.3 | 6.7 | 8.5 | 9.1 |
Sodium (mEq/L) | 135–145 | 138 | 142 | 146 | 143 |
Potassium (mEq/L) | 3.5–5.0 | 4.5 | 3.6 | 4.2 | 5.3 |
ALT (U/L) | 7–56 | 27 | 154 | 188 | 210 |
AST (U/L) | 8–48 | 69 | 118 | 67 | 100 |
ALP (U/L) | 40–129 | 96 | 233 | 166 | 161 |
Bilirubin, Total (mg/dL) | ≤1.2 | 1.8 | 1.23 | 1.1 | 1.3 |
Bilirubin, Direct (mg/dL) | ≤0.4 | 1.8 | 1.23 | 1.10 | 1.3 |
Bilirubin, Indirect (mg/dL) | ≤0.8 | 0.0 | 0.0 | 0.0 | 0.0 |
Renal and liver function tests were deranged, and the patient was oliguric. The patient was managed with IV fluids, broad spectrum antibiotics, and IV proton pump inhibitors. Provisional diagnosis of acute severe pancreatitis with multiple organ failure(Ranson score 5) was made and patient was managed along the same lines.
Oliguria and prolonged metabolic acidosis necessitated haemodialysis, and the patient was transferred to the critical care unit on day 4 of treatment. Because of severe acidosis and impaired sensorium, the patient was intubated. The tropical infection workup came out negative.
The patient’s health deteriorated further, with continuous temperature spikes, severe organ dysfunction, and shock necessitating vasopressors. Serial abdominal ultrasonography demonstrated continuous peri-pancreatic collection and a bulky pancreas. Since the patient had an invasive line and had received multiple antibiotics there was suspicion of candidemia for which the patient was started on Caspofungin empirically on day 5 of treatment. The antifungal had little effect, and the patient succumbed. Later, Candida kefyr grew in the blood fungal culture which was sent before death, and it was susceptible to fluconazole, voriconazole, amphotericin B, and caspofungin.
DISCUSSION
We report a case of severe acute pancreatitis with Candida kefyr bloodstream infection which was managed with caspofungin. Candida kefyr (formerly Candida pseudotropicalis) is a yeast with Kluyveromyces marxianus as its recognised teleomorph3,4. Candida kefyr is a new and unusual non-Candida albicans Candida species (NCAC) that has become increasingly widespread in recent years. In light microscopy elongated blastoconidia were seen arranged parallelly, like the typical “logs in a stream” appearance (Figure 1). The patient had critical illness with multiple organ failure, broad-spectrum antibiotic exposure, prolonged ICU stay with invasive lines, and renal replacement therapy, all of which mirror the immunocompromised state, prolonged hospitalization, and invasive interventions identified in the review by Dufresne SF et al9.
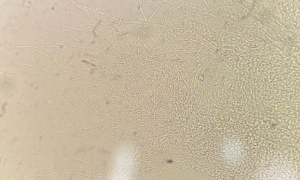
Figure 1: Potassium hydroxide (KOH) wet mount showing Candida kefyr colonies 10X. The plate was examined using 10X and 40X elongated blastoconidia were seen arranged parallelly, like the typical “logs in a stream” appearance
Fungal pathogen has been documented in both superficial and systemic infections and is identified using histology, PCR, and DNA sequencing. Infections are a major problem in immunocompromised people and recent research has shown that this organism is susceptible to many antifungals5,6,7, however, several antifungals (amphotericin B, itraconazole, voriconazole, posaconazole, fluconazole, caspofungin micafungin, and anidulafungin) have greater rates of resistance8,9,10. In our case it had shown susceptibility to fluconazole, voriconazole, and amphotericin. After 48 hours of suspecting candidemia and administering caspofungin to the patient, the patient did not respond, and a postmortem examination revealed a diagnosis of Candida kefyr candidemia. According to a study conducted at John Hopkins University, it has been observed that certain Candida kefyr species have the ability to persist as colonizers9. In immunocompromised patients, these species can lead to severe bloodstream infections8, with their pathogenicity further heightened by the formation of biofilms, similar to other Candida species11. It has demonstrated a particularly high propensity (due tocolonisation) to produce illness and, as a result, resistance against the antifungals described above, which makes treatment options more difficult to implement. Treatment options for patients with these fungal infections have expanded in recent years; however, studies have shown that delays (mostly due to C. kefyr diagnosis and identification) in initiating appropriate antifungal therapy resulted in poor clinical compliance and outcomes12. The patient’s poor response to caspofungin treatment may be attributed to acquired genetic mutations in the Candida kefyr strain. Although the isolate was reported as susceptible in vitro post-mortem, documented cases exist in which C. kefyr developed resistance during therapy (e.g., to echinocandins) and isolates with reduced susceptibility to amphotericin B have been described. This may support the possibility that in vivo resistance, biofilm formation, or drug-tolerance mechanisms may have contributed to therapeutic failure in our patient14. Additionally, there is currently no universally established minimum inhibitory concentration (MIC) threshold for determining susceptibility of Candida kefyr as outlined by the Clinical and Laboratory Standards Institute (CLSI)13. Consequently, the susceptibility of Candida kefyr to caspofungin is assessed using epidemiological cutoff (ECOFF) values. This impresses upon the importance of conducting additional research to ascertain the in vitro and in vivo susceptibility of Candida kefyr to antifungal medications. The main limitations of this report include the absence of fungal biomarker testing before post-mortem culture confirmation and the lack of follow-up imaging to assess disease progression.
In light of the emergence of this new pathogen, it is crucial to remain aware of its presence, enabling the timely initiation of appropriate antifungal treatment in cases where the initial response is unsatisfactory.
CONCLUSIONS
In light of the emergence of this new pathogen, Candida kefyr, it is crucial to remain aware of its presence in an acute severe pancreatitis patient, enabling the timely initiation of appropriate antifungal treatment. Delayed identification of such candidemia may lead to mortality. This case also highlights the importance of collecting and publishing culture reports that become available after a patient’s demise to document and disseminate such significant observations.
ACKNOWLEDGEMENTS
We thank all the personnel of our outpatient clinic, including nurses, doctors and residents. No funding was received for this research paper.
DECLARATION FOR THE USE OF GENERATIVE ARTIFICIAL INTELLIGENCE (AI) IN SCIENTIFIC WRITING:
No
INFORMED CONSENT
A written informed consent was obtained from the patient. This is a case report and no approval from the AIIMS research ethics committee was required.
CONFLICTS OF INTEREST STATEMENT
The authors declare no conflict of interest.
SOURCE OF FUNDING
None
AUTHOR’S CONTRIBUTION
JS: Contributed to data curation, investigation, formal analysis, draft writing and editing
SS: Conceptualized the study and contributed to formal analysis, resources, supervision, reviewing and editing.
IX: Contributed to the microbiological diagnosis and approved the manuscript.
MP: Contributed to supervision, resources and reviewing.
UB: Contributed to supervision, resources and reviewing.
REFERENCES
1. Baronia AK, Azim A, Ahmed A, Gurjar M, Marak RSK, Yadav R, et al. Invasive candidiasis in severe acute pancreatitis: experience from a tertiary care teaching hospital. Indian J Crit Care Med. 2017;21(1):40-5.
2. Reda NM, Hassan RM, Salem ST, Yousef RHA. Prevalence and species distribution of Candida bloodstream infection in children and adults in two teaching university hospitals in Egypt: first report of Candida kefyr. Infection. 2023;51(2):389-95.
3. Cai J, Roberts IN, Collins MD. Phylogenetic relationships among members of the ascomycetous yeast genera Brettanomyces, Debaryomyces, Dekkera, and Kluyveromyces deduced by small-subunit rRNA gene sequences. Int J Syst Bacteriol. 1996;46(2):542-9.
4. Lachance MA. Current status of Kluyveromyces systematics. FEMS Yeast Res. 2007;7(5):642-5.
5. Okmen F, Ekici H, Ari SA. Case report of a tubo-ovarian abscess caused by Candida kefyr. J Obstet Gynaecol Can. 2018;40(11):1466-7.
6. Nurdin RSC, Vitayani S, Amin S, Kadir D, Djamaluddin W, Adriani A. Cutaneous candidiasis caused by Candida kefyr. Pan Afr Med J. 2021;38:178.
7. Salehi F, Esmaeili M, Mohammadi R. Isolation of Candida species from gastroesophageal lesions among pediatrics in Isfahan, Iran: identification and antifungal susceptibility testing of clinical isolates by E-test. Adv Biomed Res. 2017;6:103.
8. Jyothi L, Reddy NP, Naaz S, Jyothi DL, Reddy N, Naaz S. An unusual case of Candida kefyr fungemia in an immunocompromised patient. Cureus. 2021;13(3):e53202. Available from: https://www.cureus.com/articles/53202-an-unusual-case-of-candida-kefyr-fungemia-in-an-immunocompromised-patient
9. Dufresne SF, Marr KA, Sydnor E, Staab JF, Karp JE, Lu K, et al. Epidemiology of Candida kefyr in patients with hematologic malignancies. J Clin Microbiol. 2014;52(6):1830-7.
10. Dagi HT, Findik D, Senkeles C, Arslan U. Identification and antifungal susceptibility of Candida species isolated from bloodstream infections in Konya, Turkey. Ann Clin Microbiol Antimicrob. 2016;15(1):36.
11. Nagy F, Bozó A, Tóth Z, Daróczi L, Majoros L, Kovács R. In vitro antifungal susceptibility patterns of planktonic and sessile Candida kefyr clinical isolates. Med Mycol. 2018;56(4):493-500.
12. Bellmann R, Smuszkiewicz P. Pharmacokinetics of antifungal drugs: practical implications for optimized treatment of patients. Infection. 2017;45(6):737-79.
13. Fekkar A, Meyer I, Brossas JY, Dannaoui E, Palous M, Uzunov M, et al. Rapid emergence of echinocandin resistance during Candida kefyr fungemia treatment with caspofungin. Antimicrob Agents Chemother. 2013;57(5):2380-2.
14. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antifungal susceptibility testing of yeasts: CLSI document M60. 2nd ed. Wayne, PA: CLSI; 2020.
Submit a Manuscript:
Copyright © Author(s) 2025. JASPI- Journal of Antimicrobial Stewardship Practices and Infectious Diseases.

