3-Step Model- An Explorative Novel Approach to Classify Sepsis: A Longitudinal Study
JASPI September 2024/ Volume 2/Issue 3
Copyright: © Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Pilania J, Das A, Panda PK, Chauhan U. 3-step Model- An Explorative Novel Approach to Classify Sepsis: A Longitudinal Study. JASPI. 2024;2(3):-15-21 DOI: 10.62541/jaspi046
ABSTRACT
Background: Sepsis remains a critical healthcare challenge worldwide, demanding prompt identification and treatment to improve patient outcomes. Given the absence of a definitive gold-standard diagnostic test, adjunct diagnostic tools are imperative to aid in early sepsis detection and guide effective treatment strategies. This study introduces a novel 3-step model to identify and classify sepsis, integrating current knowledge and clinical guidelines to enhance diagnostic precision.
Methods: This longitudinal study was conducted at a tertiary care teaching hospital in northern India. Adult patients admitted with suspected sepsis underwent screening using predefined criteria. The 3-step model consisted of assessing dysregulated host response using a National Early Warning Score-2 (NEWS-2) score of ≥6 (step 1); evaluating risk factors for infection (step 2); and assessing infection presence through clinical, supportive, or confirmatory evidence (step 3). Based on this Model, patients were categorized into asepsis, possible sepsis, probable sepsis, or confirmed sepsis at various intervals during hospitalization.
Results: A total of 230 patients were included. Initial categorization on Day 1 showed 13.0% in asepsis, 35.2% in possible sepsis, 51.3% in probable sepsis, and 0.4% in confirmed sepsis. By Day 7, shifts were observed with 49.7% in asepsis, 9.5% in possible sepsis, 25.4% in probable sepsis, and 15.4% in confirmed sepsis. At discharge or death by day 28, categories were 60.4% asepsis, 5.2% possible sepsis, 21.7% probable sepsis, and 12.6% confirmed sepsis. Transitions between categories were noted throughout hospitalization, demonstrating the dynamic nature of sepsis progression and response to treatment.
Conclusion: The 3-step Model effectively stratifies sepsis status over hospitalization, facilitating early identification and classification of septic patients. This approach holds promise for enhancing diagnostic accuracy, guiding clinical decision-making, and optimizing antimicrobial stewardship practices. Further validation across diverse patient cohorts and healthcare settings must confirm its utility and generalizability.
KEYWORDS: Asepsis, classification criteria, confirmed sepsis, possible sepsis, probable sepsis
INTRODUCTION
Sepsis is a critical, life-threatening condition resulting from a dysregulated host response to infection, leading to systemic inflammation and organ dysfunction. The term “sepsis” dates back to ancient Greece, where Hippocrates used it to describe a decaying body.1 Over time, the understanding and definition of sepsis have evolved significantly. In 1992, the American College of Chest Physicians and the Society of Critical Care Medicine established the Sepsis-1 definitions, categorizing sepsis as an inflammatory response to infection characterized by at least two Systemic Inflammatory Response Syndrome (SIRS) criteria.2 These criteria included abnormalities in heart rate, respiratory rate, body temperature, and white blood cell count.3 However, this definition was criticized for broad application, as the SIRS criteria could also reflect non-septic inflammatory responses. 4 In fact, more than 90% of ICU patients met SIRS criteria, which lacked specificity in diagnosing sepsis.5 The introduction of “severe sepsis” in the Sepsis-1 definitions aimed to denote organ dysfunction associated with sepsis. Despite this, the use of SIRS criteria persisted, leading to continued debate and the need for more precise diagnostic criteria.
In 2001, the Sepsis-2 definitions updated the sepsis criteria by incorporating the Sequential Organ Failure Assessment (SOFA) score to identify organ dysfunction more accurately. Nonetheless, the reliance on SIRS criteria for diagnosing sepsis persisted, which remained a limitation. The inability to differentiate between a normal inflammatory response and the severe response characteristic of life-threatening sepsis was a significant concern.6 In 2016, the Sepsis-3 definitions were introduced, redefining sepsis as a life-threatening organ dysfunction caused by a dysregulated host response to infection.7 This definition emphasized an acute increase of ≥2 points in the SOFA score when an infection is suspected and removed the term “severe sepsis” from clinical use.4 The new criteria aimed to improve diagnostic accuracy and focus on organ dysfunction rather than inflammatory responses alone.
Despite advances in sepsis definitions and diagnostic criteria, sepsis remains a global health challenge with high mortality rates. Various scoring systems, such as SOFA, NEWS (National Early Warning Score)9, qSOFA (quick SOFA), and APACHE-II (Acute Physiology and Chronic Health Evaluation-II), are employed to assess severity and prognosis but do not directly diagnose sepsis. Biomarkers like procalcitonin (PCT) and C-reactive protein (CRP) are also used to aid diagnosis, with PCT being more specific.10
Although diagnosing and managing sepsis has undergone a paradigm shift over the decades, we still lack a gold standard test for sepsis diagnosis. So, to aid sepsis diagnosis, a novel 3-step model was prepared, deriving from the latest available sepsis definition. The latest definition of sepsis has two major components: dysregulated host response and evidence of infection. These are combined as a step-3 process in our model for classifying sepsis.
This study aims to classify sepsis using a novel 3-step model that integrates current definitions and clinical guidelines to enhance diagnostic precision.
METHODOLOGY
Study design
This longitudinal study was designed to evaluate a novel 3-step model for sepsis classification in a real-world clinical setting. The model assesses patients on Day 1, Day 7, Day 14, and Day 28/discharge/death, evaluating shifts among categories over time.
Study setting
The study was conducted at a tertiary care teaching hospital in northern India. Data were collected from the Department of General Medicine from January 1, 2023, to December 31, 2023. The institute’s ethics committee approved the study.
Study participants
Participants included adult patients aged 18 years or older admitted to the Department of General Medicine with suspected sepsis during the study period.
Inclusion criteri
Patients aged ≥18 years
Admitted with suspected sepsis, as defined by the presence of one or more of the following criteria: need for antibiotics, evidence of infection, organ dysfunction not explained by non-infective causes, improvement following antibiotic treatment
Exclusion criteria
Patients diagnosed with an alternative condition within five days of admission.
Patients with incomplete data
Calculation of sample sizeThe study employed universal sampling because no prior reference studies were available to calculate the precise sample size. All eligible patients during the study period were included.
Data collection
Patients without evidence of dysregulated host response (step 1) were subjected to step 2 for evaluation of risk factors for infection (step 2), and patients without dysregulated host response and no evident risk factor for infection were categorized into the asepsis group (Fig 1). Patients with evidence of dysregulated host response (step 1) were directly subjected to step 3 for sepsis classification. However, risk factors for infection were also evaluated (Table 1 &2).
Figure 1: Flowchart of 3-Step Model for sepsis classification.
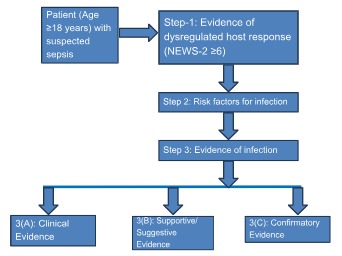
Table 1: 3-Step Model for Sepsis Classification.
Step | Description |
Step 1: Evidence of Dysregulated Host Response | Assessed using the National Early Warning Score-2 (NEWS-2) ≥6 |
Step 2: Risk Factors for Infection | Evaluated based on the presence of risk factors such as chronic illnesses, malnutrition, unhygienic living conditions, immunosuppressive states, age, trauma, structural diseases, recent surgery, travel history, animal bites, and previous hospitalizations |
Step 3: Evidence of Infection | Determined through: |
3(A) Clinical Evidence | Syndromic diagnosis including pyelonephritis, infective endocarditis, intra-abdominal infections, skin and soft tissue infections, meningitis, cerebrospinal fluid shunt infections, catheter-related infections, osteomyelitis, abscesses, and pneumonia |
3(B) Supportive/ Suggestive Evidence | Imaging (X-ray, ultrasound, computed tomography, magnetic resonance imaging, positron emission tomography) and biomarkers (blood, urine, other fluids) |
3(C) Confirmatory Evidence | Direct visualization, endoscopic evidence, microscopy and culture growth, polymerase chain reaction/gene detection, and immunological methods |
Data were collected from patient records and medical charts using RedCap software (All India Institute of Medical Sciences, Rishikesh version) and Microsoft Excel. Information collected included baseline demographics, vital signs (for NEWS-2 calculation), laboratory results, microbiological investigation data, empirically used antibiotics, ICU admission and ICU stay duration, and outcomes (discharge status and mortality)
Outcome measures
The primary outcome measure was the proportion of patients classified into different sepsis categories (asepsis, possible sepsis, probable sepsis, confirmed sepsis) on Day 1, Day 7, Day 14, and Day 28/discharge/death.
The secondary outcome measure indicated changes in sepsis categories over time.
Table 2: Criteria to consider sepsis categories/classification as per the 3-step model
S. No. | Interpretation | Outcome |
1 | (i) Step-1 = negative (ii) Step-1= positive with step-2 & 3= negative | Asepsis |
2 | Step-1, 2 & 3(a) = positive | Possible sepsis |
3 | Step-1, 2 & 3(b) = positive | Probable sepsis |
4 | Step-1, 2 & 3(c) = positive | Confirm sepsis |
Statistical analysis
In this longitudinal study, statistical analysis was performed, and the data was entered in MS Excel sheet & RedCap software (AIIMS Rishikesh version). The data were summarized using descriptive statistics. Continuous variables were presented as mean ± standard deviation (SD). Categorical variables were presented as frequencies and percentages. Cross-tabulation was used to examine the distribution of patients across different sepsis categories at each time point. The study evaluated the changes in sepsis categories over time, particularly the transition of patients from one category to another between Day 1 and subsequent time points (Day 7, Day 14, and outcome). The Stuart-Maxwell test was employed to analyze changes in sepsis categories over time. A significant Chi-square (χ²) value with a p-value < 0.05 was considered evidence of a considerable change in sepsis categorization over time. The test results were presented as χ² values with corresponding p-values for each time interval (Day 1 to Day 7, Day 1 to Day 14, Day 1 to outcome). All statistical analyses were conducted using the statistical software SPSS-25 (Statistical Package for the Social Sciences).
RESULT
A total of 1867 patients were screened, and after inclusion criteria were met., patients with missing data were excluded, resulting in a study cohort of 230 patients for analysis (Table 3). The majority of patients were young, with an equal gender distribution.
Table 3: Study characteristics and patient outcomes
Characteristics | Value | Characteristics | Value |
Age in years (Mean ± SD) | 40.70 ± 14.49 | NEWS-2 Score (Mean ± SD) | 4.08 ± 3.08 |
Age Group 18-40 years 41-60 years >60 years | No (%) 113 (49.13%) 94 (40.87%) 23 (10%) | ICU admission- N (%) | 23 (10%) |
Gender Male Female | No (%) 113 (49.1%) 117 (50.9%) | Days of ICU stay (Mean ± SD) | 10.17 ± 13.21 |
Days of hospitalization (Mean ± SD) | 11.38 ± 7.58 | Outcome- N (%) Discharged with stable vitals Discharged with unstable vitals Death | 210 (91.3%) 14 (6.1%) 6 (2.6%) |
On Day 1, the sepsis classification was dominated by probable sepsis (51.3%) and possible sepsis (35.2%), which was later comprised mainly of the asepsis (60.4%) category at the time of outcome. Figure 2 shows the proportion of patients in different categories of sepsis on Day 1, Day 7, Day 14, and Day 28/ discharge/ death (outcome).
Figure 2: Depicts the patients into different sepsis categories on different days.
Apart from overall sepsis categorization, change from one category to a different category was also evaluated with time course as the secondary outcome (Table 4-6). It was observed that from day 1 to day 7, 18.9% and 23.1% of patients belonging to probable and possible sepsis categories, respectively, moved to the asepsis category. This trend was observed throughout the course of observation.
Table 4: Change in sepsis category from Day 1 to Day 7
Category of Sepsis | Day 1 | Stuart- Maxwell test | ||||||
|---|---|---|---|---|---|---|---|---|
Asepsis | Possible Sepsis | Probable Sepsis | Confirmed Sepsis | Total | χ2 | P Value | ||
Day 7 | Asepsis | 13 (7.7%) | 39 (23.1%) | 32 (18.9%) | 0 (0.0%) | 84 (49.7%) | 91.238 | <0.001 |
Possible Sepsis | 0 (0.0%) | 6 (3.6%) | 10 (5.9%) | 0 (0.0%) | 16 (9.5%) | |||
Probable Sepsis | 2 (1.2%) | 7 (4.1%) | 34 (20.1%) | 0 (0.0%) | 43 (25.4%) | |||
Confirmed Sepsis | 0 (0.0%) | 5 (3.0%) | 21 (12.4%) | 0 (0.0%) | 26 (15.4%) | |||
Total | 15 (8.9%) | 57 (33.7%) | 97 (57.4%) | 0 (0.0%) | 169 (100.0%) | |||
The uncoloured cells on the diagonal represent patients whose category did not change. The Light blue color or other close color option represent patients who moved to a lower category, and the green-shaded cells represent patients who moved to a higher category.
Table 5: Change in sepsis category from Day 1 to Day 14
Category of Sepsis | Day 1 | Stuart- Maxwell test | ||||||
|---|---|---|---|---|---|---|---|---|
Asepsis | Possible Sepsis | Probable Sepsis | Confirmed Sepsis | Total | χ2 | P Value | ||
Day 14 | Asepsis | 2 (2.7%) | 17 (23.3%) | 21 (28.8%) | 0 (0.0%) | 40 (54.8%) | 45.153 | <0.001 |
Possible Sepsis | 0 (0.0%) | 0 (0.0%) | 2 (2.7%) | 0 (0.0%) | 2 (2.7%) | |||
Probable Sepsis | 1 (1.4%) | 2 (2.7%) | 18 (24.7%) | 0 (0.0%) | 21 (28.8%) | |||
Confirmed Sepsis | 0 (0.0%) | 2 (2.7%) | 8 (11.0%) | 0 (0.0%) | 10 (13.7%) | |||
Total | 3 (4.1%) | 21 (28.8%) | 49 (67.1%) | 0 (0.0%) | 73 (100.0%) | |||
The uncoloured cells on the diagonal represent patients whose category did not change. The red-shaded cells represent patients who moved to a lower category, and the green-shaded cells represent patients who moved to a higher category.
Table 6: Change in sepsis category from Day 1 to outcome
Category of Sepsis | Day 1 | Stuart- Maxwell test | ||||||
|---|---|---|---|---|---|---|---|---|
Asepsis | Possible Sepsis | Probable Sepsis | Confirm Sepsis | Total | χ2 | P Value | ||
At Outcome | Asepsis | 29 (12.6%) | 66 (28.7%) | 44 (19.1%) | 0 (0.0%) | 139 (60.4%) | 135.117 | <0.001 |
Possible Sepsis | 0 (0.0%) | 6 (2.6%) | 6 (2.6%) | 0 (0.0%) | 12 (5.2%) | |||
Probable Sepsis | 1 (0.4%) | 3 (1.3%) | 46 (20.0%) | 0 (0.0%) | 50 (21.7%) | |||
Confirmed Sepsis | 0 (0.0%) | 6 (2.6%) | 22 (9.6%) | 1 (0.4%) | 29 (12.6%) | |||
Total | 30 (13.0%) | 81 (35.2%) | 118 (51.3%) | 1 (0.4%) | 230 (100.0%) | |||
The uncoloured cells on the diagonal represent patients whose category did not change. The Dark green color shaped cells represent patients who moved to a lower category, and the Light green color shaped cells represent patients who moved to a higher category.
DISCUSSION
This study applied the 3-step sepsis classification model to evaluate its effectiveness in a cohort of patients with suspected sepsis till 28-day hospitalization/discharge/death, whichever is earlier. The results demonstrate the model’s utility in enhancing sepsis classification and tracking patient progress. Step 1 involves the use of the NEWS-2 score to detect dysregulated host responses indicative of sepsis when the score is ≥6 and prompts further evaluation.8 This study confirms that early identification through NEWS-2 is crucial for timely intervention and improved outcomes. This initial step is fundamental in triggering the diagnostic process and prioritizing patients for further assessment.
Step 2 evaluates the presence of risk factors that predispose patients to infections. These factors include chronic conditions, immunosuppressive states, and recent surgical procedures. By identifying patients with these risk factors, healthcare providers can better manage those at higher risk and tailor their monitoring and treatment strategies accordingly.10 Adding this step to the whole model enhances the inclusiveness of all sepsis patients as they are vital to timely treatment. Step 3 is the most comprehensive component of the model, which involves the following.
3(A) Clinical Evidence: This includes a syndromic diagnosis based on the patient’s clinical presentation. Identifying infections such as pyelonephritis or pneumonia helps narrow the possible sepsis aetiology.
3(B) Supportive/Suggestive Evidence: Incorporates imaging and biomarker analysis to provide additional evidence supporting the diagnosis. This step helps in corroborating clinical suspicions and guiding treatment decisions.
3(C) Confirmatory Evidence: Involves definitive tests such as microbiological cultures, PCR, and endoscopic findings to confirm the presence of infection. This step is crucial for establishing a definitive diagnosis and guiding targeted therapy.
In this study, the proportion of patients classified as asepsis increased from Day 1 to Day 28. This increase should be understood in the context of evolving diagnostic accuracy rather than improved management alone. Initially, many patients were misclassified due to limited information and early-stage investigations. As more comprehensive diagnostic results became available, accurate classifications emerged, reflecting the true sepsis status of patients.11
On Day 1, the majority of patients were categorized as probable sepsis (51.3%) or possible sepsis (35.2%). By Day 7, there was a notable shift, with 49.7% of patients reclassified as asepsis, demonstrating the model’s ability to track changes in patient status over time. This transition highlights the importance of ongoing assessment and the role of the 3-step model in refining sepsis classification as more information becomes available.12
The further reduction in the proportion of patients with higher sepsis severity by Day 14 and Day 28 underscores the model’s effectiveness in monitoring and managing sepsis. The increase in the asepsis category is attributed to improved diagnostic clarity rather than solely to treatment efficacy. The final assessment on Day 28 showed that 60.4% of patients were classified as having asepsis, reflecting accurate diagnosis rather than improved management alone.13
Many scoring and classification systems, simple and complex in nature, are available for sepsis globally, however, they do not incorporate other aspects of sepsis for diagnosis, such as risk factors, clinical scenarios, and the dynamic nature of sepsis, making their use limited for sepsis diagnosis, therefore, used for majorly for prognostication and mortality assessment.4 Hence, they are not true sepsis classification systems. Death due to antimicrobial resistance (AMR) is rising and will rise further to devastating levels if not controlled, as recently predicted in a global database.14 This is the right time to classify sepsis first; then only data on AMR mortality in sepsis can be predicted correctly.
Integrating the 3-step model with established tools like NEWS-2 or even SOFA score offers a comprehensive approach to sepsis management. While NEWS-2 helps identify at-risk patients, the 3-step model provides a structured framework for categorizing and monitoring sepsis progression. This combined approach enhances the accuracy of sepsis classification and supports timely interventions. This approach also tries to incorporate different aspects of sepsis into a single model, improving its real utility and usage for different population cohorts and overcoming the limitations of existing tools for identifying host responses.
The non-availability of a gold standard test for the diagnosis of sepsis creates a lacuna in early diagnosis and management, which can be filled with a more robust and structured approach, as used in this novel sepsis model. The use of this novel model for diagnosis and classification of sepsis helps the treating physician and emergency team to classify the spectrum of sepsis, from asepsis to sepsis including possible and probable sepsis, leading to improved diagnostic and therapeutic stewardship, supported with 4Ds of antimicrobial stewardship starting with the right drug.15 This initial step is crucial for sepsis patient management, reducing AMR and improving patient outcomes such as duration of hospital stay, morbidity and mortality.
Despite all measures, this study has a few limitations. Firstly, the study was conducted at a single tertiary care centre, which may limit the generalizability of the findings to a broader population. The results might reflect specific characteristics of the study site or patient population, reducing the external validity and potentially affecting the achievement of the research aims. Secondly, the 3-step approach/model prepared for this study was not previously utilized or studied in any study, making its application in real-world scenarios challenging.
CONCLUSIONS
Considering the need for the gold standard test to diagnose sepsis, the 3-step sepsis classification model demonstrates significant potential in improving sepsis diagnostic classification and management. The model contributes to better clinical outcomes and more effective use of healthcare resources by offering a structured approach to categorizing sepsis and tracking patient progress. It also demonstrated the dynamic nature of sepsis, which is relevant in terms of management and improved patient outcomes. It is promising to enhance diagnostic accuracy, guide clinical decision-making, optimize antimicrobial stewardship practices and decrease the burden of alarming AMR. However, further validation across diverse patient cohorts and healthcare settings is essential to confirm its utility and generalizability in the population.
INFORMED CONSENT
Written informed consents were obtained from patients. The confidentiality of the patients was maintained in the article.
CONFLICTS OF INTEREST STATEMENT
The authors declare no conflict of interest.
SOURCE OF FUNDING
None
AUTHOR’S CONTRIBUTION
JP: Data collection; Analysis; Writing the draft
AD: Data collection; Analysis; Writing the draft
PKP: Conceptualization; Investigation; Methodology; Resources; Review & Editing
UC: Conceptualization; Investigation; Methodology; Resources; Review & Editing
REFERENCES
Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-10.
Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250-6.
Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing Sepsis as a Global Health Priority – A WHO Resolution. N Engl J Med. 2017;377(5):414-417.
Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181-247.
Sprung CL, Sakr Y, Vincent JL, Le Gall JR, Reinhart K, Ranieri VM, Gerlach H, Fielden J, Groba CB, Payen D. An evaluation of systemic inflammatory response syndrome signs in the Sepsis Occurrence In Acutely Ill Patients (SOAP) study. Intensive care medicine. 2006 Mar;32:421-7.
Royal College of Physicians. National Early Warning Score (NEWS) : Standardising the Assessment of Acute-illness Severity in the NHS. London: Royal College Of Physicians; 2017.
Sartelli M, Kluger Y, Ansaloni L, et al. Raising concerns about the Sepsis-3 definitions. World J Emerg Surg. 2018;13:6.
Welch J, Dean J, Hartin J. Using NEWS2: an essential component of reliable clinical assessment. Clin Med (Lond). 2022;22(6):509-13.
Subbe CP, Kruger M, Rutherford P, Gemmel L. Validation of a modified Early Warning Score in medical admissions. QJM. 2001;94(10):521-6.
Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41(5):1167-74.
Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707-10.
Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372(17):1629-38.
Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323-9.
GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990-2021: a systematic analysis with forecasts to 2050. Lancet. 2024;404(10459):1199-226.
Dixit D, Ranka R, Panda PK. Compliance with the 4Ds of antimicrobial stewardship practice in a tertiary care centre. JAC Antimicrob Resist. 2021;3(3):dlab135.
Submit a Manuscript:
Copyright © Author(s) 2024. JASPI- Journal of Antimicrobial Stewardship Practices and Infectious Diseases.

