SASPI Ltd.
Implementation of a Clinical Pharmacology-Centric Antimicrobial Stewardship Initiative within COVID-19 Intensive Care Units at a Leading Tertiary Care Referral Center in India
JASPI September 2024/ Volume 2/Issue 3
Copyright: © Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Gamad N, Bhattacharjee S, Shafiq N, Bhalla A, Kajal K, Bhagat H, et al.Implementation of a Clinical Pharmacology-Centric Antimicrobial Stewardship Initiative within COVID-19 Intensive Care Units at a Leading Tertiary Care Referral Center in India JASPI. 2024;2(3):-22-28 DOI: 10.62541/jaspi042
ABSTRACT
Background: The importance of antimicrobial stewardship during the COVID-19 pandemic remains underreported.
Methods: We prospectively audited antimicrobial use in COVID-19 ICUs of a tertiary care center from April 2021 to June 2021 during the delta wave of COVID-19 in India. Prospective audits and feedback (PAF) were conducted concurrently during case discussions on all days during this period. A summary of feedback, including empiric antibiotic rationality, de-escalation, etc., was mailed to all the treating physicians every day.
Results: 161 out of 183 patients (87.97%) were empirically prescribed antibiotics in COVID ICUs, with an average of three antibiotics per patient. The most commonly prescribed empiric antibiotic was piperacillin-tazobactam, followed by ceftriaxone and colistin. De-escalation was suggested in 45.2% of prescriptions, which were followed in 36.5% but delayed in around 16% of suggestions. The presence of multiple comorbidities with intubation and/or shock was the most common reason for initiating and continuing empirical antibiotics. Around 29.8% of the patients on antibiotics developed MDR infections, and Acinetobacter baumannii was the most common organism isolated, with an average length of stay of 20 (19.6 days).
Conclusion: Antimicrobial stewardship can play an essential role in minimizing the use of unnecessary antimicrobials in COVID-19 patients.
KEYWORDS: Antibiotic, prescription, COVID-19, De-escalation, antimicrobial resistance, antimicrobial stewardship practices
INTRODUCTION
The World Health Organization (WHO) has declared the irrational use of antimicrobials and antimicrobial resistance a global emergency, considering the impact on society. Therefore, these problems require urgent action.1 The current novel coronavirus disease of 2019 (COVID-19) pandemic has further exaggerated the issue and caused unprecedented healthcare burdens due to the lack of specific medication to cure the disease. Rampant misuse of medicines, especially antimicrobials, has become a common practice, and such use has become a common concern in the healthcare community. Overlapping clinical features, critically ill status, comorbidities, hospitalization, and lack of specific and low-cost diagnostics press for antibiotic prescriptions even when not needed. A living systematic review by Langford et al. highlighted that the incidence of community- or hospital-acquired bacterial co-infection in COVID-19 patients is the same as that seen during seasonal and pandemic influenza episodes.2 In this study, the overall proportion of COVID-19 patients with bacterial infection was 6.9% (95%CI 4.3-9.5), while in critically ill patients, the incidence was 8.1% (95%CI 2.3-13.8). Alarmingly, the proportion of patients with COVID-19 who received antimicrobials was 71.9% (95%CI 56.1 to 87.7), which is disproportionate in many ways.2 In another systematic review of COVID-19 patients suffering from bacterial co-infections, Lansbury et al. reported that secondary bacterial infections were seen in 7% of hospitalized and 14% of ICU or mixed ward COVID-19 patients.3 If we look at the Indian experience with secondary bacterial infections during COVID-19, a study conducted by the Indian Council of Medical Research, ICMR revealed the prevalence of secondary infection in 3.6% of patients with COVID-19 with a predominance of gram-negative pathogens (78%) [mostly Klebsiella pneumoniae (29%) Acinetobacter baumannii (21%)] and that the mortality rate was high (56) in those patients with a secondary bacterial or fungal infection as compared to overall mortality rate (10.65%) in total admitted patients with COVID-19. They also found a high level of carbapenem resistance in the Gram-negative bacilli (GNB) mentioned above.4 These studies reiterate that bacterial co-infection is relatively less prevalent in hospitalized patients with COVID-19, and most of these patients may not require empirical antibacterial treatment with broad-spectrum agents. While searching for the reason for secondary infections in community-acquired pneumonia patients (CAP), nasopharyngeal colonizing Gram-positive organisms were found to be one of the most common causes, while for hospital-acquired or ventilator-associated pneumonia, isolates reflect the institutional antibiogram.5 Some reports also suggest secondary invasive pulmonary aspergillosis as a cause of worsening COVID-19 acute respiratory distress syndrome (ARDS).6 Referring cases with incomplete details and increased self-medication with antimicrobials such as azithromycin, ceftriaxone, and ivermectin have also contributed to the indiscriminate use of antimicrobials in this pandemic.7
Antimicrobial stewardship activities have been shown to bring about various benefits such as reduced cost of treatment, improved clinical outcome, reduced use of antimicrobials, and decreased length of hospital stay, to name a few.8 However, only a few models of antimicrobial stewardship activities during the pandemic are available in the public domain.9,10 Practice settings in low and middle-income countries (LMIC) add to the complexity of difficulty in decision-making about antimicrobials in the situation of the pandemic.8 As our health care facility has an existing antimicrobial stewardship team that comes up with models tailored to LMIC, we initiated a system for prospective audit and feedback during the second wave of the pandemic.
The aim of the present study was to report the process and outcome of antimicrobial stewardship activities initiated in intensive care units (ICUs) of a tertiary care referral centre in North India.
METHODOLOGY
Study design and population
The study was designed as a prospective study conducted in COVID hospital, Nehru Extension Block (NHE), Post Graduate Institute of Medical Education and Research (PGIMER), a tertiary care centre in Chandigarh, India, from 24 April 2021 to 24 June 2021. Our patient population included all COVID-19 patients admitted to intensive care units (ICUs) during this period (covering the second wave of COVID-19 in India).
Prescription audit
The antibiotic prescription audit was conducted daily, including holidays, during case discussions, and concurrently, feedback was given to treating physicians, essentially anaesthetists for ICUs. At the end of each day, a summary of stewardship feedback was mailed to concerned faculty members. Auditing included the collection of data from the antibiotic prescriptions, such as the following, before giving feedback:
Demographics of patient
Provisional/Definitive diagnosis
Antimicrobials prescribed along with their indication, dose, duration, frequency, route of administration, start and stop dates
Reason for starting antimicrobial- empiric, prophylactic or laboratory-based
Organ function status of the patient- liver, kidney, and lung
Factors affecting antibiotic prescriptions such as comorbidities, severity of illness, oxygen saturation, oxygen requirement, invasive procedures, previous hospital stay or referral from other wards of the hospital, and previous antibiotic prescription
Concomitant intake of steroids or, tocilizumab or any other immunosuppressants
Microbiological reports of the specimen sent for analysis
Assessing the rationale of antibiotic use required an algorithmic approach akin to the one we used previously.11 The approach was discussed by analyzing local evidence and a consensus decision.
Feedback
For the inappropriate selection of antimicrobials, we guided the right choice of antimicrobials with the right dose and duration. Broad-spectrum antimicrobials were chosen as the target area of intervention for feedback and were reviewed at 48 h of initiation. We suggested antibiotic de-escalation when laboratory reports were available, considering the clinical status and diffusion into the site of action (primarily lungs). Antibiotic de-escalation suggestions included (i) stopping antimicrobials if there was no evidence of infection, (ii) replacing empiric antimicrobials with agents with a narrower spectrum once culture and sensitivity results were available, and (iii) discontinuing antimicrobials with an overlapping spectrum of activity, for example, a combination of antimicrobials with double anaerobic coverage or double gram-negative coverage targeting same organism. We also categorized the outcome of our feedback as follows: followed with delay (if antimicrobials were de-escalated after 48 hours but before four days of suggestion) or not followed. We also suggested loading doses of drugs in critically ill patients, modifying doses in the context of renal or hepatic failure, and correcting the frequency and infusion duration of antibiotic administration. Switching from intravenous to oral therapy was also suggested when the patient improved clinically, could tolerate oral feeds, and had an appropriate oral antibiotic available with desirable bioavailability. Despite a thoughtful empiric or definitive treatment and adequate source control, if the desired response was not observed, escalation to the reserve group of antimicrobials such as linezolid, tigecycline or colistin/polymyxin B was suggested by us.
Since the COVID hospital was newly opened and a few days new beds were getting added up to account for the unprecedented input of patients during the delta wave, there was a discordance in the process of sending samples for biomarkers and culture and sensitivity and retrieval of the reports. We initiated communication between the microbiology department and anaesthetists taking care of ICUs and established a sample collection and reporting system. A messaging group comprising microbiologists (In charge of bacterial culture, susceptibility reporting and biomarker reporting) was created to track the samples sent and reports received. The report would arrive early in the messaging group, and this simple system not only streamlined the process but also fastened the treatment modification.
We noticed that antifungals were started empirically in some patients without clear indication. Hence, we established a policy for antifungal use, keeping in mind the logistics at our institution. A meeting with mycologists, anaesthetists, and physicians was arranged, and a framework for adopting the approach to antifungal use was established, based on which we can start empirical therapy and de-escalate appropriately. We presented concepts like bronchoalveolar lavage fluid sampling, KOH mount and nuances of fungal culture. We also made a framework for starting empiric antifungal therapy based on host factors, clinical suspicion, and mycological evidence.
Outcome measurement
We measured the impact of our feedback by assessing whether our suggestions were followed. We also measured clinically by collecting data on mortality, discharge, shift to step-down units, and the development of healthcare-associated infection caused by multidrug-resistant organisms (MDRO) or fungus.
Statistical analysis
We have provided descriptive statistics of our feedback by expressing the outcomes in the percentage of suggestions followed, mortality, discharge, shift to other wards, and development of healthcare-associated infection caused by MDROs.
RESULTS
Characteristics of the patient population
161 patients out of 169 (95.3%) were put on at least one systemic antibiotic in ICUs from 24 April 2021 to 24 June 2021. The mean age of the included patients was 50.6 years, ranging from 20 to 91 years. 60.2% (97 out of 161) were males and 39.7% (64 out of 161) were females. These patients’ average length of stay in the hospital was 20 (19.6 days). 58% of the patients had one or the other comorbidities such as hypertension, diabetes mellitus, obesity, hypothyroidism, previous coronary artery bypass grafting (CABG), and old age, among others. 60% of the patients were under mechanical ventilation with intubation or tracheostomy. 45% of the patients had shock and were under inotropic support, 31% had either renal or hepatic dysfunction, 20% of the patients received steroids for more than ten days, 24% had a history of prior hospitalization or referral from other wards of this institute and 9% had a previous history of antibiotic intake (Table 1).
Table 1: Characteristics of the study population | |
Mean age (years) | 50.6 |
Male gender (%) | 60.2 |
The average length of hospital stay (days) | 19.6 |
The average number of antimicrobials prescribed per patient | 3 (2.596) |
Clinical characteristics (%)
|
58 60 31 45 24 20 9 |
We identified that dexamethasone was the most commonly prescribed steroid (87.5%), followed by methylprednisolone (14.2%). Tocilizumab was used as an immunosuppressant in 40 cases (23.6%).
Antimicrobial prescriptions
There were a total of 418 antimicrobial prescriptions for 161 patients (Table 1), averaging 3 (2.6 to be precise) per patient. While most use was empiric, lab-based use was 16.9% of the total antimicrobial use. The most common antimicrobials prescribed empirically were piperacillin-tazobactam, followed by ceftriaxone, colistin, meropenem, vancomycin, azithromycin, and teicoplanin. Other antimicrobials that were prescribed were amikacin, amoxicillin-clavulanic acid, antitubercular drugs, cefoperazone-sulbactam, clindamycin, doxycycline, imipenem-cilastatin, metronidazole, minocycline, polymyxin B, etc. based on culture and sensitivity report (Figure 1, Table 2). The most common antifungals included liposomal amphotericin B, voriconazole, caspofungin, and cotrimoxazole (Table 2). Double gram-negative covers were identified in 35 cases (8.37%), whereas double anaerobic covers were identified in 11 cases (2.63%). The most common “Reserve” group of drugs prescribed empirically was colistin (13.2%), followed by polymyxin B and minocycline. The total percentage of empiric use of reserved drugs was 14.2%.
Figure 1: Percentage prescription of Antimicrobials
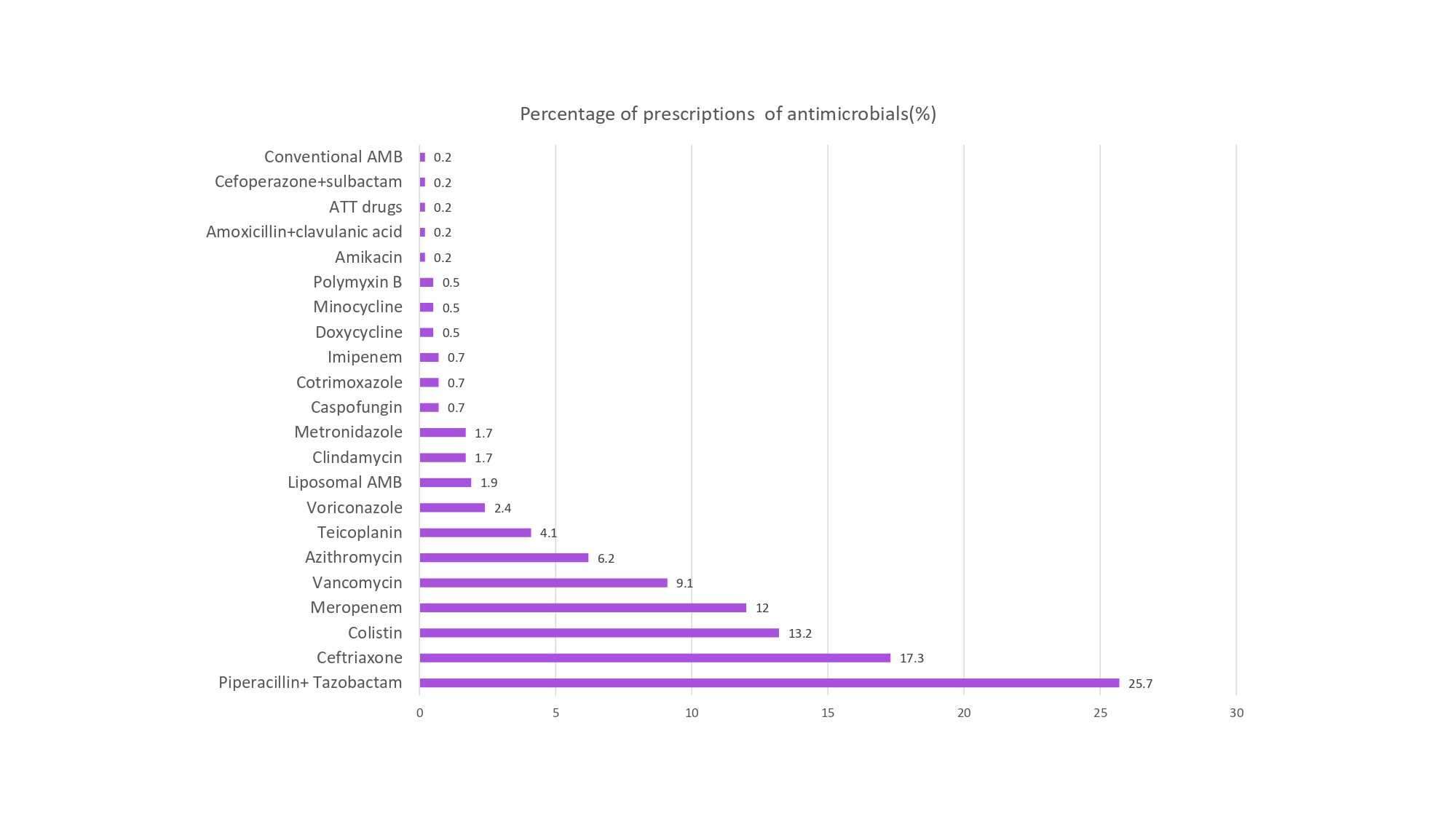
Table 2: Percentage of antimicrobial prescriptions | |
Antimicrobials | Percentage of prescriptions (%) |
Piperacillin + tazobactam | 25.7 |
Ceftriaxone | 17.3 |
Colistin | 13.2 |
Meropenem | 12 |
Vancomycin | 9.1 |
Azithromycin | 6.2 |
Teicoplanin | 4.1 |
Voriconazole | 2.4 |
Liposomal AMB | 1.9 |
Clindamycin | 1.7 |
Metronidazole | 1.7 |
Caspofungin | 0.7 |
Cotrimoxazole | 0.7 |
Imipenem | 0.7 |
Doxycycline | 0.5 |
Minocycline | 0.5 |
Polymyxin B | 0.5 |
Amikacin | 0.2 |
Amoxicillin+clavulanic acid | 0.2 |
ATT drugs | 0.2 |
Cefoperazone+sulbactam | 0.2 |
Conventional AMB | 0.2 |
We suggested de-escalation in 189 prescriptions, which was followed in 36.5% (69 out of 189), followed by delay in 15.34% (29 out of 189), and not followed in 48.15% (91 out of 189) of the cases (Figure 2). De-escalation of ceftriaxone and azithromycin was the most commonly followed suggestion. In the case of empiric antifungals, de-escalation was suggested in 3 cases (6.2%), followed in 2 cases, and followed with delay in one case. Presence of multiple comorbidities, including renal or hepatic dysfunction, respiratory failure, hypertension, diabetes, hypothyroidism, obesity and previous CABG, shock, previous hospitalization or referral from other wards, prior intake of antimicrobials invasive procedures such as intubation and mechanical ventilation, central venous lines, peripheral lines, urinary catheters and tracheostomy and co-prescription of steroids or other immunosuppressants were the reasons for continued empiric antibiotic use. Intubation with or without tracheostomy, the presence of any comorbidities, and shock were the most common reasons for continuing empiric therapy despite recommendations for stopping or de-escalation. The reasons for continuation are listed in (Table 3) as given by the treating team.
Figure 2: Pattern of outcomes for de-escalation suggestion made by the Antimicrobial stewardship team.

Table 3: Reason for continued empiric therapy | |
Reasons for continued empiric treatment despite de-escalation suggestion | Percentage (%) |
Intubation with or without tracheostomy | 24.3 |
Comorbidities | 23.5 |
Shock | 18.2 |
Organ dysfunction | 12.6 |
Prolonged use of Steroids | 8.1 |
Admission to another hospital | 6.5 |
Prior intake of antimicrobials | 3.6 |
Patterns of distribution of MDR organisms
The incidence of growth of multidrug organisms in patients was 29.8%. The maximum culture positivity rate was with endotracheal aspirates. Overall, the identified organisms were multidrug-resistant Acinetobacter baumannii (22.9%). Other organisms grown were Klebsiella pneumoniae, Pseudomonas aeruginosa, Escherichia coli, Enterobacter cloacae, Chryseobacterium gleum, Streptococcus pneumoniae, Enterococcus faecium, Staphylococcus hemolyticus, Staphylococcus epidermidis, Staphylococcus hominis, Acinetobacter nosocomialis, species of Elizabethkingia, Stenotrophomonas maltophilia, Pseudomonas stuzeri, Burkholderia cepecia, Achromobacter xylosoxidance and Ochrobactrum anthropi. Whenever organisms such as Staphylococcus epidermidis, Staphylococcus hemolyticus and Staphylococcus hominis were grown, we advised repeat testing following sterile sampling techniques.
Regarding fungal infections, 32 patients (19%) had some evidence (presence of yeast cells, fungal biomarkers raised) of fungal infections. Of that, 11 cases (6.7%) were treated based on fungal biomarkers alone, and 58.6% did not have any evidence of fungal infection. For 21% of patients, the reports were not available.
Follow-up and clinical outcome
We also determined if the organism grew within 72h or later to ascertain if these organisms were contaminants. 19.75% of the patients developed HAI with fungi as diagnosed by fungal culture or serum biomarkers such as beta-D-glucan or galactomannan index or KOH mount. The site of fungal infection included either urinary tract, lungs or blood. We followed up with 161 patients, and 56.25% of these patients died in COVID-19 ICUs, rest were discharged. Since a single patient received multiple antimicrobials and the de-escalation suggestion/ ASP intervention was directed to a single antibiotic, the comparative analysis between mortality and de-escalation of antimicrobials could not be assessed.
DISCUSSION
Our study highlights that an active antimicrobial stewardship program can be instrumental in streamlining the use of antimicrobials even during the pandemic situation. The study was conducted in a tertiary care facility designated as a referral centre. Most referred patients required supportive management and received antimicrobials, which were often broad-spectrum. Further, several patients did not come with adequate laboratory and radiological/imaging workup at the time of admission. In their review, Kanz SS et al. listed the reasons for high empiric antimicrobial use in COVID-19 patients. The clinical picture, radiographic evidence simulating bacterial pneumonia, and patients admitted to ICU with septic shock-like features were few to name [5]. Besides this, there was a hike in invasive devices, especially ventilators extracorporeal membrane oxygenation, contributing to the fear of MDRO and a surge in empiric antimicrobial use. Ippolito M et al., in their systematic review and meta-analysis of studies from the United Kingdom, Europe, Russia, and China, found that patients with COVID-19 are at significantly greater risk of VAP than those intubated but without COVID-19.12 The reason for this specific finding might be related to the very nature of COVID-19 which is a multisystem disorder. Further, at the time of the delta wave, the use of corticosteroids for the management of patients was increasingly being resorted to, and often this use was deviant from the recommended use.13
While there was conflicting evidence regarding the prevalence of MDRO during the COVID-19 pandemic, a retrospective cohort study by Palanysamy et al. found that the prevalence of carbapenem-resistant BSI (bloodstream infection) was 47.2%, of which Acinetobacter baumannii was isolated in most cases followed by Klebsiella pneumoniae.3 Although the incidence of MDR-GNB infections was less in our study as compared to the mentioned study, the pattern of isolated organisms was similar, It was difficult to assess whether the patients had infection with the isolated organisms at the time of admission to our facility as very few patients had these details in the referral samples. Microbiological samples were sent only if there was a need for guiding therapy. Since several patients required invasive lines, dialysis, intubation, broad-spectrum antimicrobials, and a prolonged stay in the hospital, acquired infections occurring during stay in the unit can also not be ruled out. However, best infection control practices with the pandemic’s limitations were being followed.
Our study noted a similar prevalence of empiric antibiotic use with piperacillin-tazobactam as the highest-prescribed empiric antibiotic, followed by ceftriaxone and colistin. Notably, empiric colistin use was in considerable percentage, probably because of the baseline prevalence of carbapenem-resistant organisms even during the non-pandemic period. Although our feedback was followed in a little over half of the suggestions, still a large proportion of feedback was not followed. The reasons for reluctance to de-escalate were primarily dictated by the patient profile, which largely centred around evidence of organ failure. In the retrospective cohort study by Lakbar I et al., a decline in antibiotic de-escalation rate (ADE) was noted compared to non-COVID-19 patients (ADE 52.2% vs 27.6% in non-covid and covid-19 groups, respectively). The authors justified that the fear of losing a patient and anxiety related to the pandemic resulted in such a decline.14
We could not evaluate the impact of de-escalation on mortality. A retrospective study suggested that clinical outcomes, including mortality, hospital stay, and ICU readmission rates, did not differ between the antibiotic de-escalated and the continued antibiotic group. The authors concluded that antibiotic de-escalation did not increase mortality in critically ill patients.14
This pandemic made clear that this virus will stay there for quite some time in the respiratory microbiome ecosystem and, from time to time, re-appear like outbreaks incorporating new genetic changes and resistance mechanisms. This leads us to develop more robust, integrated, and evidence-driven ASPs where a holistic one-health approach will be established.15,16 The resurgence of MDROs post-pandemic with limited availability of newer antimicrobials in LMICs leads us to square one, which is “prevention is better than cure”.
CONCLUSION
Antimicrobial stewardship can play an important role in minimizing the use of unnecessary antimicrobials in COVID-19 patients. This must be supplemented with pharmacokinetic-pharmacodynamic considerations and biomarker-driven decisions. Beyond the pandemic, without newer antimicrobials in hand, ASP is the only way and should be strengthened to fight the next probable catastrophe—MDROs.
ACKNOWLEDGEMENT
None
CONFLICTS OF INTEREST STATEMENT
The authors declare no conflict of interest.
SOURCE OF FUNDING
None
AUTHOR’S CONTRIBUTION
NG: Conceptualization; Data collection; Analysis; Writing the draft
SB: Conceptualization; Data collection; Literature review; Analysis; Writing the draft
NS: Conceptualization; Methodology; Validation; Supervision; Review & Editing
AB, KK, HB, AH, VM, KS, AS, SM, GKM, RG, NNB, KS, NK, IB, NV, AA, IS, and PDG: Methodology; Data collection; Writing the draft
REFERENCES
Coronavirus disease (COVID-19) pandemic. https://www.who.int/europe/emergencies/situations/covid-19
Langford BJ, So M, Raybardhan S, et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infect. 2021;27(4):520-31.
Zirpe KG, Tiwari AM, Gurav SK, et al. Timing of Invasive Mechanical Ventilation and Mortality among Patients with Severe COVID-19-associated Acute Respiratory Distress Syndrome. Indian J Crit Care Med. 2021;25(5):493-8.
Palanisamy N, Vihari N, Meena DS, et al. Clinical profile of bloodstream infections in COVID-19 patients: a retrospective cohort study. BMC Infect Dis. 2021;21(1):933.
Vijay S, Bansal N, Rao BK, et al. Secondary Infections in Hospitalized COVID-19 Patients: Indian Experience. Infect Drug Resist. 2021;14:1893-1903.
Nori P, Cowman K, Chen V, et al. Bacterial and fungal co-infections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect Control Hosp Epidemiol. 2021;42(1):84-8.
Kanj SS, Ramirez P, Rodrigues C. Beyond the Pandemic: The Value of Antimicrobial Stewardship. Front Public Health. 2022;10:902835.
Daria S, Islam MR. Indiscriminate Use of Antibiotics for COVID-19 Treatment in South Asian Countries is a Threat for Future Pandemics Due to Antibiotic Resistance. Clin Pathol. 2022;15:2632010X221099889.
Pierce J, Apisarnthanarak A, Schellack N, et al. Global Antimicrobial Stewardship with a Focus on Low- and Middle-Income Countries. Int J Infect Dis. 2020;96:621-9.
Assi M, Abbas S, Nori P, et al. Infection Prevention and Antimicrobial Stewardship Program Collaboration During the COVID-19 Pandemic: a Window of Opportunity. Curr Infect Dis Rep. 2021;23(10):15.
Song M, Deng Z, Chan O, Grépin KA. Understanding the Implementation of Antimicrobial Policies: Lessons from the Hong Kong Strategy and Action Plan. Antibiotics (Basel). 2022;11(5):636.
Kakkar AK, Shafiq N, Sahni N, et al. Assessment of Appropriateness of Antimicrobial Therapy in Resource-Constrained Settings: Development and Piloting of a Novel Tool-AmRAT. Antibiotics (Basel). 2021;10(2):200.
Ippolito M, Misseri G, Catalisano G, et al. Ventilator-Associated Pneumonia in Patients with COVID-19: A Systematic Review and Meta-Analysis. Antibiotics (Basel). 2021;10(5):545.
Lakbar I, Delamarre L, Curtel F, et al. Antimicrobial Stewardship during COVID-19 Outbreak: A Retrospective Analysis of Antibiotic Prescriptions in the ICU across COVID-19 Waves. Antibiotics (Basel). 2022;11(11):1517.
Aldardeer NF, Shukairi ANAL, Nasser ME, et al. Continuation versus de-escalation of broad-spectrum antibiotic therapy in critically ill COVID-19 patients. Dr Sulaiman Al Habib Med J. 2023;5:33-41.
Schouten J, De Waele J, Lanckohr C, et al. Antimicrobial stewardship in the ICU in COVID-19 times: the known unknowns. Int J Antimicrob Agents. 2021;58(4):106409.
Submit a Manuscript:
Copyright © Author(s) 2024. JASPI- Journal of Antimicrobial Stewardship Practices and Infectious Diseases.

