Diversity in Fungal Infections in the Initial Waves of COVID-19: An Occurrence Based Systematic Review
JASPI June 2024/ Volume 2/Issue 2
Copyright: © Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
April-June 30, 2024
Sharma S, Singh A, Banerjee T. Diversity in Fungal Infections in the Initial Waves of COVID-19: An Occurrence Based Systematic Review. JASPI. 2024;2(2)page no. JASPI. 2024;2(2)18-38 DOI: 10.62541/jaspi020
ABSTRACT
Background: Fungal coinfections have been part of the complications in coronavirus disease (COVID-19) patients. While systematic reviews on individual fungus was available, comprehensive data on the occurrence of various fungal infections was limited.
Methodology: A systematic search in the databases ‘PubMed’ and ‘Global Research Database on COVID-19 by the World Health Organization (WHO) was made using relevant search terms. Only fungal coinfections/superinfections in confirmed COVID-19 cases were considered. All observational studies, case series, and case reports in English were included. Overall, the occurrence of the fungal infections and the associated factors was noted. Chi-square and Fisher’s exact tests compared epidemiological factors between survived and dead.
Results: Data from 126 eligible studies reporting 870 cases showed that mucormycosis was the most common infection (42.5%), followed by aspergillosis (32.41%) and candidiasis (22.87%). The majority of the infections were seen in severe COVID-19 (94.01%), in ICU (67.25%), and with mechanical ventilation (73.61%). Prior steroid therapy was seen in 81.3% in mucormycosis. In aspergillosis, mechanical ventilation, infection due to Aspergillus fumigatus and administration of steroids at more than the recommended dose were significantly associated with those who died (p<0.05).
Conclusion: Mucormycosis, followed by invasive pulmonary aspergillosis and invasive candidiasis, has been the most common coinfections/superinfections in COVID-19 patients. Early diagnosis led to better survival in Covid associated mucormycosis (CAM); however, in COVID-19-associated pulmonary aspergillosis (CAPA), mechanical ventilation, larger doses of corticosteroids than recommended and infection with A. fumigatus were significant associations among those who succumbed to the condition.
KEYWORDS: Aspergillosis; Candidiasis; Coinfections/superinfections; COVID pandemic; Epidemiological factors; Mucormycosis; Steroid
INTRODUCTION
The coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) brought a plethora of other related complications in the susceptible hosts. With the limitless hospital admissions due to the virus, a massive increase in healthcare-associated infections (HAI) with opportunistic pathogens was witnessed in the form of several bacterial and fungal infections.1,2 There have been several original studies and reviews on this aspect of coinfections in COVID-19 patients. 3, 4 The best example of this crisis was the unprecedented rise in mucormycosis cases during the second wave of the pandemic in India.5 Studies have shown several predisposing risk factors associated with the fungal coinfections.6, 7
While most of the systematic reviews have focused on a single fungal pathogen,8-10, this systematic review was performed to get comprehensive data on the various fungal infections across the globe in COVID-19 patients published in literature since the beginning of the pandemic.
METHODOLOGY
Search strategy
A systematic search was performed in two electronic databases ‘PubMed’ and ‘Global research database on COVID-19 by the World Health Organization (WHO)’. Articles published between January 01, 2019, to June 15, 2021 were searched by using the following search terms: The PubMed database was searched using advanced search tool with the MeSH terms entered as (((((((((((Covid-19) OR (COVID-19)) OR (Coronavirus disease)) ) AND (Fungal diseases)) OR (Fungal coinfections)) OR (Secondary fungal infections)) OR (Covid associated mucormycosis)) OR (Mucormycosis)) OR (Candidiasis)) OR (Aspergillosis)) OR (Aspergillus), while the WHO database was searched using the search terms (Covid-19 OR COVID-19 OR Coronavirus disease) AND (Fungal diseases OR Fungal coinfections OR Secondary fungal infection OR Covid associated mucormycosis OR Mucormycosis OR Candidiasis OR Aspergillosis OR Aspergillus).
Definitions and standard recommendations
Coinfection was defined as the recovery of another respiratory fungal pathogen along with SARS-COV-2 when COVID-19 was diagnosed. Superinfection was defined as the subsequent recovery of pathogens during COVID-19 treatment and care.4 The severity of COVID-19 was classified according to WHO standards.11 WHO recommended choice for corticosteroids in severe COVID-19 only at recommended doses of 32mg methylprednisolone per day or its equivalent (6mg dexamethasone per day or 50mg hydrocortisone every 8 hours) for 7 – 10 days in severe COVID-19 was taken as reference.12 Microbiological evidence of fungal infections in the form of microscopy, culture, molecular methods, and serological assays were noted to support a diagnosis of infection.
Inclusion and Exclusion criteria
All articles in the English language on fungal
coinfections or secondary fungal infections in patients with COVID-19 were included. Cases of COVID-19 confirmed by Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) only were considered.
Reviews, editorials, opinions, and research protocols without primary data were excluded. Articles with missing data on patient profiles and other variables were not considered. Reports of fungal infection without microbiological evidence of infection and reports of fungal colonization were excluded. Any retrospective data without mentioning the precise classification of the fungal isolation procedure was not included.
All grades of COVID-19-associated pulmonary aspergillosis (CAPA) by the European Organization for Research and Treatment of Cancer (EORTC) were considered for aspergillosis infections.13
Study selection
Two independent authors (AS and SS) screened the abstract of the studies. Two authors (TB and AS/SS) reviewed the full texts of the selected abstracts for eligibility. Any discrepancy or ambiguity was resolved by consensus. References cited by the articles and articles citing the selected articles were also checked for eligibility. Reporting was done according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for systematic reviews.14 The review protocol was registered in the PROSPERO register (CRD42021286172).
Data extraction
The two independent reviewers (TB and AS/SS) extracted data from the selected studies using a Microsoft Excel spreadsheet. Data was doubly checked for accuracy.
The following data was retrieved from all the studies, as per availability, study design, period/year of study, publication date, country of origin, number of cases studied/reported, age, gender, ICU admission, mechanical ventilation, history of diabetes mellitus, hypertension, obesity, malignancy, chronic obstructive pulmonary disease (COPD), number of days of fungal infection post-COVID-19, COVID-19 severity, fungal diagnosis, mode of diagnosis, fungal species, bacterial coinfection, steroid therapy, type of steroid therapy, dose of steroids used, average days of steroid therapy, prior antibiotic therapy, antibacterial treatments, antifungal treatments and outcome.
Data synthesis and Statistical analysis
The primary outcome assessed was the proportions of fungal infections reported as coinfections or superinfections in COVID-19 and their associated factors. Data was also stratified according to the global distribution of these reports in three six monthly intervals since the inception of the pandemic, i.e. January – June 2020, July – December 2020 and January – June 2021.
Heterogeneity (I2) was graded on high, substantial, moderate and low levels based on the I2 values ranging from 75-100%, 50-90%, 30-60% and below 40%, respectively.15 The funnel plot was used to evaluate the publication bias (Annexure 1). Categorial variables were expressed as relative frequencies and proportions while continuous variables as mean or median with dispersion using Medcalc® statistical software (version: 19.6.3.0)
Further, for those infections like aspergillosis and mucormycosis with sufficient data (≤ 5 parameters), clinical characteristics were compared between the survived and the dead with the Chi-square test and Fisher’s exact test in Medcalc® statistical software (version: 19.6.3.0). While calculating the overall occurrence, fungal cases with mixed infections were also included.
RESULTS
Screening of studies
A total of 1777 search articles were screened, of which 126 articles finally qualified for systematic review. The flowchart for the selection of the studies is shown in Figure 1.
Figure 1: PRISMA flowchart showing selected studies
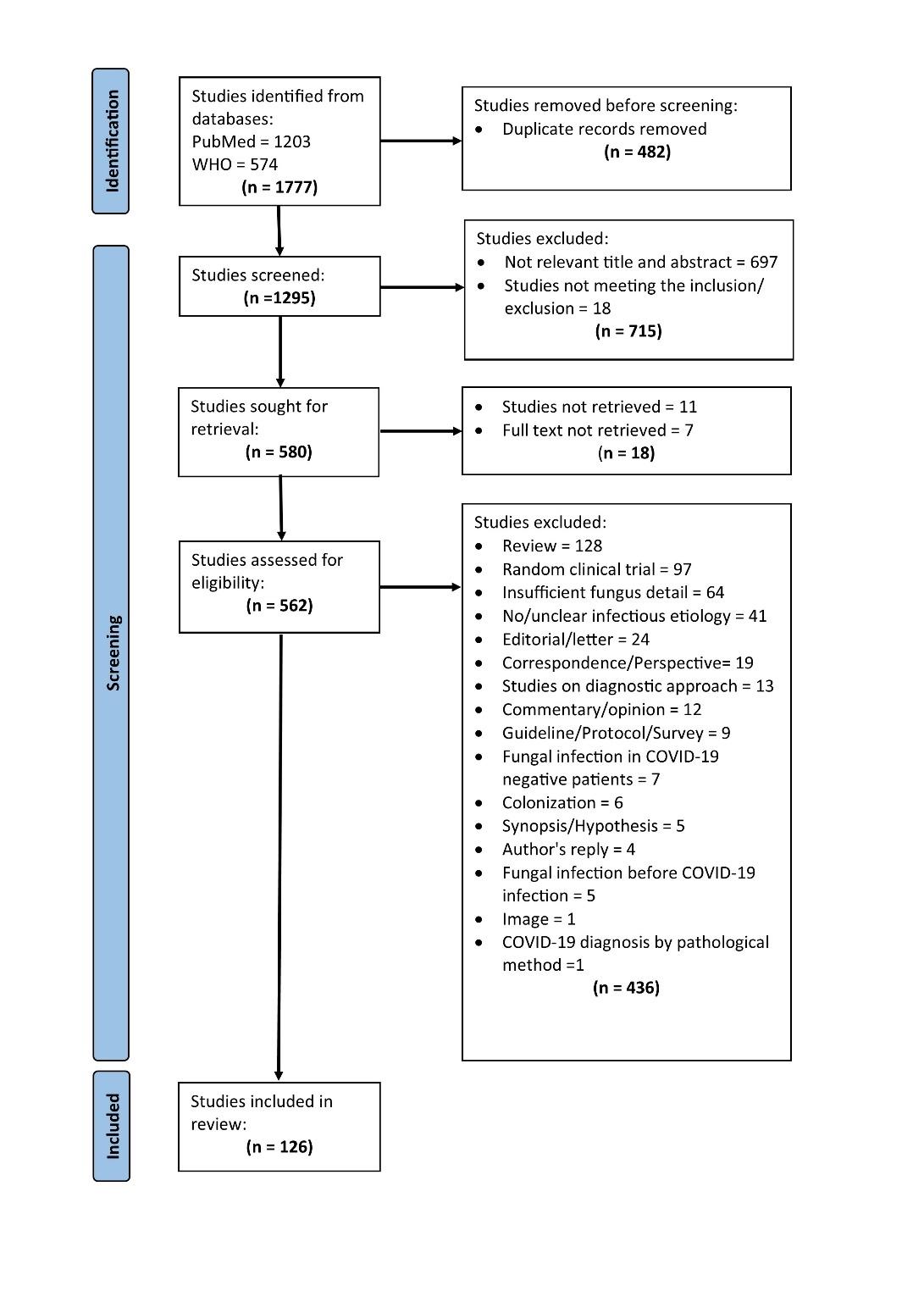
A total of 126 studies constituted 870 cases of fungal infections. 16-141
The studies included in the analysis comprised Case reports (n=84), Cross-sectional studies (n=19), Cohort studies (n=11), Case series (n=8), Case-control studies (n=3) and Abstract only (n=1). The lists of included and excluded studies have been shown in Annexure 2 and Annexure 3.
These were distributed as 48 studies comprising 220 cases, 45 studies comprising 424 cases, and 33 studies comprising 226 cases in the three study periods. The majority of fungal infections were reported from France (7), the United States (11) and India (9) in January – June 2020, July – December 2020 and January – June 2021 periods, respectively. The distribution of the studies of different fungal infections in different study periods is shown in Figure 2.
Figure 2: Distribution of studies (A: Jan-2020 to Jun-2020; B: Jul-2020 to Dec-2020; C: Jan-2021 to Jun-2021) of different fungal infections in COVID-19 patients across the globe
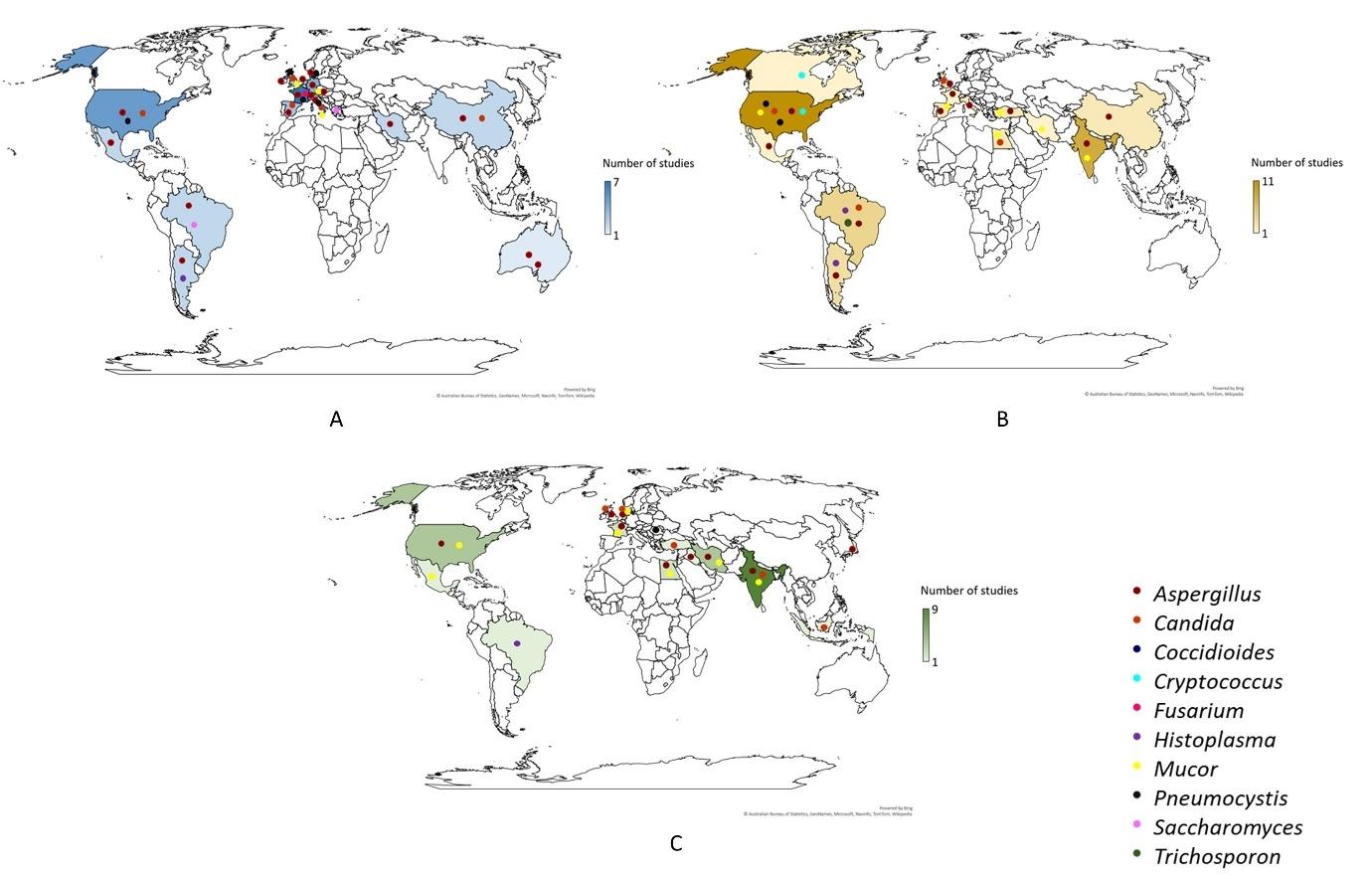
Occurrence of fungal infections
A total of 10 different types of fungal infections in COVID-19 were reported. The overall summary of epidemiological factors of fungal infections in COVID-19 patients has been shown in Table 1.
Table 1: Overall prevalence summary of epidemiological factors of fungal infections in COVID-19 patients
Parameter | Studies (N) | Sample size(N) | Positive cases (n) | Occurrence % (95% CI) |
Total | 126 | 870 | 870 | – |
Male | 110 [16-30,32-38,40-44,46-59,61-64,68-69,71-82,84-106,108,111-112,114-116,119-121,123-141] | 549 | 370 | 67.39 (63.30-71.31) |
Female | 110 [16-30,32-38,40-44,46-59,61-64,68-69,71-82,84-106,108,111-112,114-116,119-121,123-141] | 550 | 180 | 32.72 (28.82–36.82) |
Mucor | 37 [16-45,105,115-116,118-121] | 870 | 370 | 42.52 (39.22-45.89) |
Aspergillus | 63 [46-99,101,105-106,115-116,118-121] | 870 | 282 | 32.41 (29.31–35.64) |
Candida | 18 [100-114,122-124] | 870 | 199 | 22.87 (20.12-25.81) |
Pneumocystis | 7 [126-132] | 870 | 16 | 1.83 (1.05-2.97) |
Histoplasma | 4 [133-136] | 870 | 5 | 0.57 (0.19-1.34) |
Trichosporon | 1 [141] | 870 | 5 | 0.57 (0.19-1.34) |
Saccharomyces | 2 [123,139] | 870 | 3 | 0.34 (0.07-1.00) |
Coccidioides | 1 [137] | 870 | 1 | 0.11 (0.00-0.60) |
Cryptococcus | 2 [125,138] | 870 | 2 | 0.22 (0.03-0.83) |
Fusarium | 1 [140] | 870 | 1 | 0.11 (0.00-0.60) |
ICU admission | 100 [16-28,34-39,41-44,46-48,50-57,81,85,87-88,91-101,103-109,111-113,115-116,119-120,123-128,130-139,141] | 568 | 382 | 67.25 (53.21 –60.76) |
Mechanical Ventilation | 77 [16-17,19-22,25-26,28,36-39,41-44,46-48,52,54-68,71,73-77,79-81,84,87,90-91,93-98,103-104,106-107,111-112,115,119-120,123-124,126,130-133,135-137,139-141] | 398 | 293 | 73.61 (69.0 – 77.88) |
Severe COVID-19 status | 78 [16-21,24-25,27-28,38-39,41-43,46-49,53-57,60-69,72-74,85-86,88,91,93-94,96,98,100-109,111,114-116,119,124-128,130-135,137-141] | 384 | 361 | 94.01 (91.15 – 96.) |
Diabetes | 120 [16-30,32-44,46-109,111-116,119-121,123-141] | 635 | 253 | 39.84 (36.01 – 43.77) |
Hypertension | 120 [16-30,32-44,46-48,90-109,111-116,118-121,123-141] | 653 | 266 | 40.73 (36.94 – 44.62) |
COPD | 119 [16-30,32-44,46-75,77-88,90-109,111-116,118-121,123-141] | 652 | 91 | 13.95 (11.39 – 16.86) |
Obesity | 120 [16-30,32-44,46-62,64-82,84,86-88,90-100,102-109,111-116,118-121,123-141] | 625 | 81 | 12.96 (10.43 – 15.85) |
Malignancy | 117 [16-22,24-30,32-44,46-84,86-88,90-100,102-109,111-116,118-121,123-141] | 626 | 46 | 7.34 (5.43 – 9.68) |
Bacterial coinfections | 78 [16-25,28,30,33,40-41,43-44,46-58,60,63,66,71-72,74,76-77,81,92-101,103,106,108-112,115,119-120,124-130,132-141 | 426 | 40 | 9.38 (6.79 – 12.57) |
Antibiotic treatment | 97 [16-22,24,28,30,32-33,35-37,41-44,46-59,61-64,68-69,71-77,79-82,84,87-91,93-97,99-100,102-104,106,108-116,118-120,123-130,132-141] | 452 | 272 | 60.17 (55.50 – 64.72) |
Antifungal treatment | 101 [16-22,24-28,30,32,34-44,46-64,68-69,71-75,79-81,86-89,92-96,99-100,102-107,109-112,114-116,119-121,123-141] | 388 | 303 | 78.09 (73.64 – 82.11) |
Steroid therapy | 102 [16-26,28,30,32-37,41-44,46-63,66,68-69,71-81,84,87-90,92,111-116,118-121,124-141] | 572 | 324 | 56.64 (52.47 – 60.75) |
Survived | 104 [16-21,23-28,30,32-44,46-58,60-64,66, 68-69,71-75,77-82,84,86,88,90-100,102,104-107,111-112,114-116,119-121,123-141] | 426 | 217 | 50.93 (46.08 – 55.78) |
Died | 104 [16-21,23-28,30,32-44,46-58,60-64,66, 68-69,71-75,77-82,84,86,88,90-100,102,104-107,111-112,114-116,119-121,123-141] | 426 | 209 | 49.06 (44.22 – 53.92) |
Phenotypic (Microscopy, Culture, Autopsy, Biopsy) | 98 [16-22,25-28,30,32,34-43,46-59,61-64,66-69,71-72,75-78,80-81,84,87-96,99-101,103-105,107-109,111,113-116,119-121,124-131,133-141] | 492 | 398 | 80.89 (77.14 – 84.28) |
Molecular (PCR, MALDI-TOF) | 98 [16-22,25-28,30,32,34-43,46-59,61-64,66-69,71-72,75-78,80-81,84,87-96,99-101,103-105,107-109,111,113-116,119-121,124-131,133-141] | 492 | 50 | 10.16 (7.64 –13.18) |
Immunological/Serological | 98 [16-22,25-28,30,32,34-43,46-59,61-64,66-69,71-72,75-78,80-81,84,87-96,99-101,103-105,107-109,111,113-116,119-121,124-131,133-141] | 492 | 232 | 47.15 (42.67 – 51.67) |
ICU: Intensive care unit; COPD: Chronic obstructive pulmonary disease; MALDI-TOF: Matrix-Assisted Laser Desorption/Ionization-Time Of Flight; [references of studies included in proportion analysis
Among the fungal infections, the proportion of mucormycosis cases was highest (370, 42.5%), followed by aspergillosis (282, 32.4%) and candidiasis (199, 22.8%). Other fungal pathogens reported were Pneumocystis jiroveci (16, 1.8%), Histoplasma capsulatum (5, 0.5%), Trichosporon asahii (5, 0.5%), Saccharomyces spp. (3, 0.3%), Cryptococcus neoformans (2, 0.2%), Fusarium proliferatum (1, 0.1%), and Coccidioides immitis (1, 0.1%).
The majority of the cases of fungal infections were seen in patients with severe COVID-19 (361, 94%) with ICU admission (382, 57%) and on mechanical ventilation (293, 73.6%). Nearly 40% of the cases had diabetes mellitus (253/635) and hypertension (266/653). Steroid therapy as a part of COVID-19 treatment was given in 56.6% (324/572) cases. Dexamethasone was the most commonly used agent (77, 52.02%). Bacterial coinfection was seen in only 8.7% (40/426), but antibiotic treatment was given in 60.1% (272/452) cases. Multimodal diagnostic methods were used for fungal infections with a maximum employing microscopy and culture (398, 80.8%). The data on individual fungal infections are as follows:
Mucormycosis
Thirty-one studies reported a single infection due to COVID-19-associated mucormycosis (CAM), adding up to 329 cases. Among these, rhino orbital ocular mucormycosis was seen in 321 (97.56%) cases, pulmonary in 5 (1.51%) cases, and 1 (0.3%) each of disseminated gastrointestinal and skeletal. The prevalence summary of epidemiological factors of Mucor in CAM has been included in Table 2.
Table 2: Prevalence summary of epidemiological factors of Mucor in COVID-19 associated mucormycosis (CAM)
Parameter | Studies | Sample size (N) | Positive cases (n) | Occurrence % (95% CI) |
Male | 27 [16-30,32-38, 40-44] | 91 | 65 | 71.42 (61.00-80.41) |
Female | 27 [16-30,32-38, 40-44] | 91 | 26 | 28.57 (19.59-39.00) |
ICU admission | 23 [16-28,34- 39,41-44] | 94 | 24 | 25.53 (17.09-35.37) |
Severe COVID-19 status | 15 [16-21,24-25,27-28,38-39,41-43] | 50 | 34 | 68.00 (53.30-80.48) |
Mechanical Ventilation | 17 [16-17,19-22,25-26,28,36-39,41-44] | 66 | 23 | 34.84 (23.53-47.58) |
Diabetes | 28 [16-30, 32-44] | 101 | 81 | 80.19 (71.09-87.46) |
Hypertension | 28 [16-30, 32-44] | 101 | 45 | 44.55 (34.66-54.78) |
COPD | 28 [16-30, 32-44] | 101 | 5 | 4.95 (1.63-11.18) |
Obesity | 27 [16-22,24-30,32-44] | 78 | 3 | 3.84 (0.80-10.83) |
Malignancy | 27 [16-22,24-30,32-44] | 78 | 4 | 5.12 (1.41-12.61) |
Steroid therapy | 23 [16-26,28, 30, 32-37,41-44] | 86 | 70 | 81.39 (71.55-88.98) |
Survived | 25 [16-21, 23-25,27-28, 30, 32-44] | 93 | 58 | 62.36 (51.72-72.21) |
Died | 25 [16-21, 23,25,27-28, 30, 32-44] | 93 | 35 | 37.63 (27.79-48.28) |
ICU: Intensive care unit; COPD: Chronic obstructive pulmonary disease; [references of studies included in proportion analysis]
Most cases were reported from India in the study period Jan-Jun 2021. The mean age of the patients was 55.99 ± 0.07 years, and male to female ratio was 2.5:1. Among the patients with mucormycosis, ICU admission (24, 25.5%), severe COVID-19 (34, 68%), history of diabetes (81, 80.1%), hypertension (45, 44.5%) and malignancy (4, 5.1%) was seen. Steroid use during COVID-19 in these cases was seen in 81.3% (70/86) of the cases. Mortality was reported in 37.6% (35/93) of the cases.
It was interesting to note that cases of mucormycosis were reported in Australia, Italy and the UK (3 studies) during the initial period of the pandemic, which gradually increased to 8 studies from Brazil, Egypt, Spain, the USA, Iran and Turkey during the period Jul-Dec 2020. Finally, 13 studies were found in the Jan-Jun 2021 period, with the maximum cases reported from India (278,75.13%). The majority of the studies did not mention the species of Mucor.
On comparison of the demographic and associated factors of those who survived and died with CAM, it was found that though cases with ICU admission (11, 20.37%), severe COVID-19 (16, 37.20%), mechanical ventilation (6, 13.04%), hypertension (15, 19.48%) was found more among those who died, prevalence of diabetes mellitus was comparable in both the groups (Table 3).
Table 3: Comparison of epidemiological and clinical characteristics of patients with COVID-19-associated mucormycosis (CAM) (Survived v/s Died)
Parameters | Total | Survived (%) | Died (%) | P value |
Number | 79 | 39 (49.36) | 38 (48.10) | |
Not mentioned | 2 (2.5) | |||
Demographic data | ||||
Age | 77 | 56.05 ±14.01 | 55.94 ±12.26 | 1.00 |
Male | 58 | 28 (48.27) | 30 (51.72) | 0.46 |
Female | 19 | 11 (57.89) | 8 (42.10) | |
Risk factors | ||||
ICU admission | 54 | 4 (7.40) | 11 (20.37) | 0.13 |
Not mentioned | 19 | 11 (57.89) | 8 (42.10) | |
Severe COVID-19 status | 43 | 10 (23.255) | 16 (37.20) | 0.53 |
Not mentioned | 34 | 20 (58.82) | 14 (41.17) | |
Mechanical ventilation | 46 | 2 (4.34) | 6 (13.04) | 0.43 |
Not mentioned | 31 | 20 (64.51) | 11 (35.48) | |
Diabetes | 64 | 24 (37.5) | 23 (35.93) | 1.00 |
Not mentioned | 13 | 7 (53.84) | 6 (46.15) | |
Hypertension | 77 | 12 (15.58) | 15 (19.48) | 0.47 |
COPD | 1 (1.29) | 4 (5.19) | 0.19 | |
Malignancy | 1 (1.29) | 3 (3.89) | 0.35 | |
Renal Failure | 4 (5.19) | 7 (9.09) | 0.34 | |
Chronic sinusitis | 1 (1.29) | 0 | 1.00 | |
Bacterial coinfection | 46 | 1 (2.17) | 1 (2.17) | 1.00 |
Not mentioned | 31 | 19 (61.29) | 12 (38.70) | |
Antibiotic treatment | 45 | 8 (17.77) | 12 (26.66) | 0.76 |
Not mentioned | 32 | 19 (59.37) | 13 (40.62) | |
Antifungal treatment | 76 | 35 (46.05) | 33 (43.42) | 0.71 |
Azoles | 3 (3.94) | 2 (2.63) | 1.00 | |
Echinocandin | 0 | 2 (2.63) | 0.24 | |
Amphotericin-B | 35 (46.05) | 31 (40.78) | 0.34 | |
Not mentioned | 1 | 0 | 1 (100) | |
Steroid therapy | 73 | 27 (36.98) | 29 (39.72) | 1.00 |
Not mentioned | 4 | 4 (100) | 0 | |
<10 days | 32 | 6 (18.75) | 11 (34.37) | 1.00 |
Not mentioned | 45 | 24 (53.33) | 21 (46.66) | |
>10 days | 32 | 2 (6.25) | 6 (18.75) | 0.22 |
Not mentioned | 45 | 24 (53.33) | 21 (46.66) | |
More than the recommended dose | 11 | 2 (18.18) | 5 (45.45) | 1.00 |
Not mentioned | 66 | 36 (54.54) | 30 (45.45) | |
Infection days post COVID infection | ||||
Coinfection | 7 | 5 (71.42) | 2 (28.57) | 0.22 |
Average number of days | 40 | 20.94 ±18.73 | 20.80 ±18.32 | 1.00 |
Not mentioned | 30 | 17 (56.66) | 13 (43.33) | |
ICU: Intensive care unit; COPD: Chronic obstructive pulmonary disease
However, the diagnosis of CAM as coinfection with COVID-19 was more (5, 71.42%) in those who survived.
Aspergillosis
For aspergillosis, a total of 282 cases were reported in 63 studies. Studies on aspergillosis were distributed throughout the globe during the entire pandemic. The prevalence summary of epidemiological factors of Aspergillus in CAPA has been included in Table 4.
Table 4: Prevalence summary of epidemiological factors of Aspergillus in COVID-19-associated pulmonary aspergillosis (CAPA)
Parameter | Studies | Sample size (N) | Positive cases (n) | Occurrence % (95% CI) |
Male | 49 [46-59,61-64,68-69,71-99] | 213 | 150 | 70.42 (63.80-76.46) |
Female | 49 [46-59,61-64,68-69,71-99] | 213 | 63 | 29.57 (23.54-36.20) |
ICU admission | 44 [46-48,50-77,81,85,87-88,91-99] | 210 | 180 | 85.75 (80.24-90.15) |
Severe COVID-19 status | 30 [46-49,53-57,60-69,72,74,85-86,88,90-91,93-94,96,98] | 120 | 118 | 98.33 (94.11-99.80) |
Mechanical Ventilation | 39 [46-48,52,54-68,71,73-77,79-81,84,87,89-91,93-98] | 208 | 201 | 96.63 (93.19-98.64) |
Diabetes | 54 [46-99] | 248 | 85 | 34.27 (28.39-40.54) |
Hypertension | 54 [46-99] | 248 | 118 | 47.58 (40.23-53.99) |
COPD | 53 [46-75,77-99] | 247 | 45 | 18.21 (13.61-23.61) |
Obesity | 52 [46-62, 64-84,86-99] | 245 | 48 | 19.59 (14.81-25.12) |
Malignancy | 53 [46-84, 86-99] | 246 | 20 | 8.13 (5.04-12.28) |
Steroid therapy | 41 [46-63,66,68-69,71-72,75-81,83-84,87-90,92,94,96-98] | 203 | 126 | 62.06 (55.01-68.77) |
Survived | 45 [46-58,60-64,66,68-69,71-75,77-84,86,88-96,98-99] | 179 | 71 | 39.66 (32.44-47.23) |
Died | 45 [46-58,60-64,66,68-69,71-75,77-84,86,88-96,98-99] | 179 | 108 | 60.33 (52.77-67.56) |
ICU: Intensive care unit; COPD: Chronic obstructive pulmonary disease; [references of studies included in proportion analysis]
Among the affected, a very high prevalence of mechanical ventilation (201, 96.6%), ICU admission (180, 85.7%) and use of corticosteroids (126, 62%) was seen. Most (118, 98.3%) patients had severe COVID-19 infection. A mortality of 60.3% (108/179) was seen. When data for those who survived and died due to aspergillosis infection was compared, mechanical ventilation was significantly associated with mortality (p<0.05). Besides, other factors like ICU admission, COVID-19 severity, hypertension, and diabetes mellitus were more frequent in patients who died (Table 5).
Table 5: Comparison of epidemiological and clinical characteristics of patients with COVID-19-associated pulmonary aspergillosis (CAPA) (Survived v/s Died)
Parameters | Total | Survived (%) | Died (%) | P value |
Subjects | 109 | 37 (33.94) | 61 (55.96) | |
Not mentioned | 11 (10.28) | |||
Demographic data | ||||
Age | 98 | 63.09 ±13.16 | 63.54 ±13.20 | 0.71 |
Male | 76 | 29 (38.15) | 47 (61.84) | 0.87 |
Female | 22 | 8 (36.36) | 14 (63.63) | |
Risk factor | ||||
ICU admission | 80 | 25 (31.25) | 40 (50.00) | 1.00 |
Not mentioned | 18 | 6 (33.33) | 12 (66.66) | |
Severe COVID-19 status | 62 | 26 (41.93) | 33 (53.22) | 0.58 |
Not mentioned | 36 | 9 (25.00) | 27 (75.00) | |
Mechanical ventilation | 89 | 27 (30.33) | 41 (46.06) | 0.00 |
Not mentioned | 9 | 9 (100) | 0 | |
Diabetes | 98 | 16 (16.32) | 18 (18.36) | 0.19 |
Hypertension | 17 (17.34) | 30 (30.61) | 0.83 | |
COPD | 3 (3.06) | 12 (12.24) | 0.15 | |
Obesity | 8 (8.16) | 12 (12.24) | 0.80 | |
Malignancy | 1 (1.02) | 6 (6.12) | 0.24 | |
Chronic kidney disease | 2 (2.02) | 3 (3.06) | 1.00 | |
Dyslipidaemia | 3 (3.06) | 7 (7.14) | 0.73 | |
Species | ||||
A. fumigatus | 59 | 20 (33.89) | 24 (40.67) | 0.03 |
A. niger | 0 | 4 (6.77) | 0.28 | |
A. flavus | 1 (1.69) | 2 (3.38) | 1.00 | |
A. terreus | 0 | 4 (6.77) | 0.28 | |
A. penicillioides | 0 | 1 (1.69) | 1.00 | |
A. ochraceus | 0 | 1 (1.69) | 1.00 | |
A. lentulus | 0 | 1 (1.69) | 1.00 | |
A. proliferans | 1 (1.69) | 0 | 0.37 | |
Not mentioned | 39 | 15 (25.42) | 24 (61.53) | |
Bacterial coinfection | 70 | 5 (7.14) | 14 (20.00) | 0.17 |
Not mentioned | 28 | 8 (28.57) | 20 (71.42) | |
Antibiotic treatment | 98 | 19 (19.38) | 30 (30.61) | 1.00 |
Azithromycin | 7 (7.14) | 11 (11.22) | 1.00 | |
Piperacillin-tazobactam | 6 (6.12) | 8 (8.16) | 0.76 | |
Carbapenem | 4 (4.08) | 13 (13.26) | 0.27 | |
3rd generation cephalosporins | 6 (6.12) | 12 (12.24) | 0.79 | |
Antifungal treatment | 91 | 37 (40.65) | 50 (54.94) | 0.14 |
Voriconazole | 24 (26.37) | 40 (43.95) | 1.00 | |
Isavuconazole | 2 (2.19) | 2 (2.19) | 0.63 | |
Echinocandin | 4 (4.39) | 7 (7.69) | 1.00 | |
Amphotericin-B | 8 (8.79) | 9 (9.89) | 0.41 | |
Not mentioned | 7 | 0 | 7 (100) | |
Steroid therapy | 87 | 18(20.68) | 38 (43.67) | 0.25 |
Not mentioned | 11 | 5 (45.45) | 6 (54.54) | |
<10 days | 35 | 7(20.00) | 16 (45.71) | 1.00 |
>10 days | 3 (8.57) | 9 (25.71) | 1.00 | |
More than the recommended dose | 6 (17.14) | 23 (65.71) | 0.04 | |
Not mentioned | 63 | 27 (42.85) | 36 (57.14) | |
Coinfection | 8 | 4 (50.00) | 4 (50.00) | 0.71 |
Average number of days post COVID-19 | 40 | 15.79 ±12.40 | 15.53 ±12.00 | 1.00 |
Not mentioned | 23 | 6 (26.08) | 17 (73.91) | |
ICU: Intensive care unit; COPD: Chronic obstructive pulmonary disease
Among the species, Aspergillus fumigatus (A. fumigatus) was significantly the dominant species in these infections (p = 0.03). Antifungal and presumptive antibiotic treatment was more frequent in those who died. However, the use of steroids in COVID-19 at greater than recommended doses was significantly seen in those who succumbed to the infection (p=0.04). Aspergillosis infection post-COVID-19 infection (days) was comparable in both groups.
Candidiasis
For infection with Candida spp. in COVID-19 patients, 15 studies were eligible, reporting 199 cases. The prevalence summary of epidemiological factors of Candida in COVID-19-associated candidiasis (CAC) has been shown in Table 6.
Table 6: Prevalence summary of epidemiological factors of Candida in COVID-19-associated candidiasis (CAC)
Parameter | Studies (N) | Sample size (N) | Positive cases (n) | Occurrence % (95% CI) |
Male | 10 [100-105,108,111-112,114] | 150 | 89 | 59.33 (51.02-67.27) |
Female | 10 [100-105,108,111-112,114] | 150 | 60 | 40.00 (32.10-48.31) |
ICU admission | 14 [100-113] | 186 | 156 | 83.87 (77.78-88.85) |
Severe COVID-19 status | 14 [100-112, 114] | 183 | 173 | 94.53 (90.18-97.35) |
Diabetes | 15 [100-114] | 187 | 48 | 25.66 (19.57-32.55) |
Hypertension | 15 [100-114] | 187 | 73 | 39.03 (32.00-46.43) |
COPD | 15 [100-114] | 187 | 26 | 13.90 (9.29-19.70) |
Obesity | 15 [100-114] | 187 | 19 | 10.16 (6.23-15.41) |
Malignancy | 15 [100-114] | 187 | 17 | 9.09 (5.39-14.16) |
C. albicans | 12 [100-104,106-107,109-113] | 74 | 50 | 67.56 (55.68-78.00) |
C. tropicalis | 12 [100-104,106-107,109-113] | 74 | 5 | 6.75 (2.23-15.07) |
C. glabrata | 12 [100-104,106-107,109-113] | 74 | 6 | 8.10 (3.03-16.82) |
C. parapsilosis | 12 [100-104,106-107,109-113] | 74 | 5 | 6.75 (2.23-15.07) |
C. auris | 12 [100-104,106-107,109-113] | 74 | 4 | 5.40 (1.49-13.27) |
Steroid therapy | 15 [100-114] | 187 | 82 | 43.85 (36.62-51.20) |
Antibiotic treatment | 13 [100-104,106,108-114] | 163 | 122 | 74.84 (67.40-81.30) |
Antifungal treatment | 15 [100-114] | 187 | 45 | 24.06 (18.13-30.84) |
Azoles | 15 [100-114] | 187 | 32 | 17.11 (12.01-23.29) |
Echinocandin | 15 [100-114] | 187 | 24 | 12.83 (8.40-18.49) |
Amphotericin-B | 15 [100-114] | 187 | 3 | 1.60 (0.33-4.62) |
Survived | 9 [100, 102, 104-107, 111-112,114] | 56 | 28 | 50.00 (36.34-63.66) |
Died | 9 [100, 102, 104-107, 111-112,114] | 56 | 27 | 48.21 (34.66-61.97) |
ICU: Intensive care unit; COPD: Chronic obstructive pulmonary disease; [references of studies included in proportion analysis]
Among these, 185 (92.9%) cases had bloodstream infections (BSI), while 4 (2%) cases had respiratory tract infections.Two cases reported oral infection, and one case involved urinary tract infection. The major spp. was C. albicans (50, 67.5%), followed by C. glabrata (5, 8.1%), C. tropicalis (5, 6.7%), C. parapsilosis (5, 6.7%) and C. auris (4, 5.4%).
The majority of the cases had severe COVID-19 infection and had ICU admission (94.5% and 83.8%, respectively). Prior steroid therapy was seen in 43.8% (82/187) cases. Among the different antifungal treatments, azoles (32, 17.1%) followed by echinocandin (24, 12.8%) were the most commonly used agents. The data regarding the duration and dose of steroids and other comorbidities was not available. Patients’ data on mechanical ventilation in case of candidiasis infection was also insufficient. Survival was 50% (28/56) in these infected cases.
Pneumocystosis
A total of 7 studies with 16 cases of Pneumocystis jiroveci (P. jiroveci) infection were reported, among which 3/15 (20%) cases were associated with human immunodeficiency virus (HIV). In contrast, 12/15 (80%) cases were not associated with HIV. Among the cases, the majority (11 cases, 68.7%) survived. Of the 16 cases, 10 (62.5%) cases were incidentally diagnosed using P. jiroveci-specific primers in BAL samples of COVID-19 patients.
Histoplasmosis
For histoplasmosis in COVID-19 patients, five studies reported 5 cases comprising three males and two females with a mean age of 34.8 years. None had a history of ICU admission for diabetes mellitus or hypertension. An average of 52.3 days were noted post-COVID-19 in patients with histoplasmosis. Only 2 (40%) patients received prior steroid therapy. Among them, 2 out of 4 (50%) cases were associated with HIV. All the cases were treated with itraconazole with or without amphotericin B, and there was 100% survival.
Trichosporonosis
A study from Brazil reported 5 cases of bloodstream infection with Trichosporon asahii (T. asahii) in patients with severe COVID-19. Fungal infection in these patients was seen after a mean of 22.4 days post-COVID-19 infection. Of the 5 cases, 4 were males, and the average age was 70.2 years. All were mechanically ventilated in the ICU and had received steroid therapy for an average of 22.2 days. Nearly 80% (4/5) had a fatal course.
Saccharomyces infection
Two studies reported 3 cases of Saccharomyces infection with oral lesions in one and BSI in the other two. All three cases were hypertensive males, with one also being diabetic, with a mean age of 72 years. All had been mechanically ventilated and had a history of ICU admission. The BSI was seen post Saccharomyces supplementation. The average day for fungal infection post-COVID-19 was 28 days. All were treated with fluconazole with or without anidulafungin; survival was 100%.
Cryptococcosis
This fungus was reported in 2 patients with COVID-19 in 2 studies. Both were 29-year-old males, one with HIV and diagnosed as coinfection with COVID-19, while the other with autoimmune hemolytic anaemia (AIHA) was diagnosed 27 days post-COVID-19. Unfortunately, the second patient died even though both had moderate COVID-19.
Others
Single cases of infection with Fusarium proliferatum and Coccidioides were reported in 2 different reports in the COVID-19 patients. Fusariosis was seen in a 57-year-old male diabetic, hypertensive and obese case of severe COVID-19 after nine days of COVID-19 diagnosis. Coccidioidomycosis was seen as a coinfection with COVID-19 without any comorbidity but with heart failure. Both patients survived.
DISCUSSION
Diverse fungal infections were seen in association with COVID-19 globally, as evidenced by this systematic review. While aspergillosis and invasive candidiasis have been the most common fungal coinfection or superinfection worldwide,142, the unprecedented emergence of mucormycosis, especially in the Indian context, accounted for the predominance of mucormycosis cases in COVID-19 patients. This review showed that among the published reports, the prevalence of mucormycosis cases was the highest (42.5%), with 278 cases (75.13%) reported from India.
Several factors have been implicated in the increase in fungal coinfection in COVID-19 patients, also noted in the present study. Previous systematic reviews have already hinted towards the excessive use of antibacterial agents without evidence of coinfections. 143, 144 The use of corticosteroids, previously advocated for any viral pneumonia, was found to be associated with several secondary fungal infections.30
CAM has been reported to have a lower frequency than other COVID-19-associated fungal infections unless reports from India indicate its importance.142 It was considered a ‘notifiable disease’ and earned an epidemic status in many states of India.2, 145 It is evident that CAM was restricted to the Indian subcontinent rather than involving the entire world. The initial period (first wave) of the pandemic in India was also associated with an increase in twice the number of mucormycosis cases as against the pre-COVID-19 period.5 Two potential factors that public health experts opined had contributed to the full-fledged rise of these cases were the injudicious and excessive usage of corticosteroids, both in the hospitals and the community, in COVID-19 treatment in the ‘diabetes capital’ of the world.146 A systematic review of mucormycosis in India revealed uncontrolled hyperglycemia due to the enormous burden of the diabetic population, as well as dysregulation due to COVID-19 infection was one of the critical factors for this infection.146 The most common species of Mucor was not revealed in this study due to a lack of data from most of the studies. However, in the Indian context, Rhizopus arrhizus has been reported to be the predominant pathogenic species in CAM.5 The diagnosis was more difficult owing to the challenges in isolating the fungus and lack of clinical suspicion.30 Consequently, this study revealed that a significant proportion (71.42%) of those who survived CAM infection presented as coinfection, enabling early diagnosis.
Among other fungal infections, invasive pulmonary aspergillosis (IPA) in COVID-19 patients caused an increased burden of morbidity and mortality.8 The development of CAPA resulted from interplay between different factors of the epidemiological triad,6,8 including mechanical ventilation, infection due to A. fumigatus spp. and exposure to corticosteroids at higher than the recommended dose, as revealed by this study.
Until mucormycosis emerged, invasive candidiasis was reported as the second most common fungal infection in COVID-19 patients. There has been evidence of a deranged immune response against C. albicans, the commonest reported spp., in COVID-19 patients, increasing their susceptibility to invasive candidiasis.147 Invasive candidiasis accounts for 19-40% of mortality in hospitals, often as high as 70% in ICU.148 Among the non-albicans species, C. glabrata infection was the commonest. However, even C. auris infections have been reported in long-term care (LTC) facilities, which might be related to the limited resources required for adequate maintenance of infection control practices.142
There have been issues regarding wrong diagnosis and mismanagement of P. jiroveci infection during COVID-19 due to similarity in presentation.149 Pneumocystis infection is otherwise commonly associated with immunocompromised patients, particularly with HIV infection.150 Interestingly, of the reported cases, only 20% cases were in HIV patients. The presence of P. jiroveci infections in 80% of the COVID-19-infected patients is indirect evidence of the immune dysregulation caused by the virus.
The major challenge with fungal pneumonia, such as histoplasmosis, blastomycosis, and coccidioidomycosis, is the similarity in the presentation of COVID-19 pneumonia and its timely diagnosis. Previous data had suggested that COVID-19 patients with corticosteroid therapy and HIV infections are commonly associated with histoplasmosis.1 Incidentally, of the affected patients, 40% had received prior steroid therapy and 50% were associated with HIV.
Trichosporon has been considered a re-emerging fungal pathogen following the excessive use of echinocandins in managing invasive fungal infections.151,152 Once the second commonest non-Candida cause of bloodstream infection due to any fungi in malignancy, this pathogen has shown its propensity for fatal infection in COVID-19 patients.153 Among 870 cases of fungal infections, a single study reported 5 cases of BSI with T. asahii from Brazil.152 All had a history of ICU admission. The recognized risk factors, like invasive mechanical devices in the form of central venous catheter (CVC) and mechanical ventilation, were present in all the cases. Additionally, exposure to broad-spectrum antibiotics without any evidence of bacterial coinfection initially and exposure to steroid therapy was also present, emphasizing the importance of judicious use of antibiotics, antifungals and steroids. The majority (4/5) also had BSI due to Candida spp before or simultaneously with this pathogen. As evident elsewhere, a significant risk factor for invasive Trichosporon is unregulated diabetes mellitus or hyperglycaemia, seen in 40% of the cases in the present study.153
There have been several reports of possible mechanisms and challenges in immune responses in the form of ‘storm’ with enormous cytokines release and ‘virus-driven hyperinflammation’ against the SARS-CoV2.154 Consequently, known pathogens like Cryptococcus neoformans have also taken the opportunity to infect the COVID-19 infected susceptible host.
Other less frequently reported fungal infections, like those with Saccharomyces, Fusarium and Coccidioides, were seen in COVID-19 patients with underlying comorbidity conditions and known risk factors. The only positive outcome in these infections was the 100% survival rate.
This review provides a comprehensive overview of 10 different fungal infections reported in COVID-19 patients and their epidemiological factors. However, the article had few limitations. We could not associate all the factors with regard to all the fungal infections due to paucity and lack of uniformity in the data in the reported studies. Additionally, due to a lack of data, both descriptive and observational studies were considered, accounting for substantial data variations. Data on essential factors like biochemical and immunological parameters concerning COVID-19 infection, dose and duration of steroids for all the cases, and use of other therapeutic options like immunosuppressants and immunomodulators could not be analyzed due to insufficiency. The considerable heterogeneity noted for a few of the epidemiological factors could be accounted for by the variations in the number of study subjects reported in each study, as the majority of the selected studies were case reports. Nonetheless, we endeavoured to provide information on the global prevalence of fungal infections in COVID-19 patients and the factors commonly associated with the common infections. Lastly, this comprehensive review brought forward several issues and challenges that could have led to the emergence of these fungal coinfections.
CONCLUSION
Based on published data in major electronic databases, mucormycosis, followed by invasive pulmonary aspergillosis and invasive candidiasis, have been the most prevalent coinfections/superinfections in COVID-19 patients. While in CAM, survival was better in those diagnosed early, in CAPA, mechanical ventilation, a larger dose of corticosteroids than recommended and infection with A. fumigatus were significant associations among those who succumbed to the condition. It is essential to provide the best medical practices to tackle these infections. Future studies should suggest innovative strategies to tackle such infections and the pandemic.
CONFLICTS OF INTEREST STATEMENT
The authors declare no conflict of interest.
SOURCE OF FUNDING
None
AUTHORS’ CONTRIBUTION
SS: Literature Search; Data Extraction; Data Analysis; Manuscript Writing
AS: Literature Search; Data Extraction
TB: Conceptualization, Supervision; Data Extraction; Review & Editing
REFERENCES
Roudbary M, Kumar S, Kumar A, Černáková L, Nikoomanesh F, Rodrigues CF. Overview on the Prevalence of Fungal Infections, Immune Response, and Microbiome Role in COVID-19 Patients. J Fungi (Basel). 2021; 7(9):720.
Rocha ICN, Hasan MM, Goyal S, et al. COVID-19 and mucormycosis syndemic: double health threat to a collapsing healthcare system in India. Trop Med Int Health. 2021; 26(9):1016-8.
Feldman C, Anderson R. The role of co-infections and secondary infections in patients with COVID-19. Pneumonia (Nathan). 2021; 13(1):5.
Musuuza JS, Watson L, Parmasad V, Putman-Buehler N, Christensen L, Safdar N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS One. 2021; 16(5):e0251170.
Chakrabarti A. The recent mucormycosis storm over Indian sky. Indian J Med Microbiol. 2021; 39 (3):269-70.
Ezeokoli OT, Gcilitshana O, Pohl CH. Risk Factors for Fungal Co-Infections in Critically Ill COVID-19 Patients, with a Focus on Immunosuppressants. J Fungi (Basel). 2021; 7(7):545.
Prakash H, Skiada A, Paul RA, Chakrabarti A, Rudramurthy SM. Connecting the Dots: Interplay of Pathogenic Mechanisms between COVID-19 Disease and Mucormycosis. J Fungi (Basel). 2021; 7(8):616.
Apostolopoulou A, Esquer Garrigos Z, Vijayvargiya P, Lerner AH, Farmakiotis D. Invasive Pulmonary Aspergillosis in Patients with SARS-CoV-2 Infection: A Systematic Review of the Literature. Diagnostics (Basel). 2020; 10 (10):807.
Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India. Diabetes Metab Syndr. 2021; 15(4):102146.
John TM, Jacob CN, Kontoyiannis DP. When Uncontrolled Diabetes Mellitus and Severe COVID-19 Converge: The Perfect Storm for Mucormycosis. J Fungi (Basel). 2021; 7(4):298.
WHO. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance 13 March 2020. World Health Organization; 2020.Accessed September 20, 2021.https://iris.who.int/handle/10665/331446
World Health Organization. Corticosteroids for COVID-19 Living Guidance. World Health Organization; 2020. Accessed September 20, 2021.https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1.
Verweij PE, Rijnders BJA, Brüggemann RJM, et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med. 2020; 46 (8):1524-35.
Page MJ, McKenzie JE, Bossuyt PM, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol. 2021; 134:103-12.
Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. John Wiley & Sons; 2019.
Kanwar A, Jordan A, Olewiler S, Wehberg K, Cortes M, Jackson BR. A Fatal Case of Rhizopus azygosporus Pneumonia Following COVID-19. J Fungi (Basel). 2021; 7 (3):174.
Zurl C, Hoenigl M, Schulz E, et al. Autopsy Proven Pulmonary Mucormycosis Due to Rhizopus microsporus in a Critically Ill COVID-19 Patient with Underlying Hematological Malignancy. J Fungi (Basel). 2021; 7 (2):88.
Krishna V, Morjaria J, Jalandari R, Omar F, Kaul S. Autoptic identification of disseminated mucormycosis in a young male presenting with cerebrovascular event, multi-organ dysfunction and COVID-19 infection. ID Cases. 2021; 25:e01172.
Karimi-Galougahi M, Arastou S, Haseli S. Fulminant mucormycosis complicating coronavirus disease 2019 (COVID-19). Int Forum Allergy Rhinol. 2021; 11 (6):1029-30.
Arana C, Cuevas Ramírez RE, Xipell M, et al. Mucormycosis associated with COVID-19 in two kidney transplant patients. Transpl Infect Dis. 2021; 23(4):e13652.
Meshram HS, Kute VB, Chauhan S, Desai S. Mucormycosis in post-COVID-19 renal transplant patients: A lethal complication in follow-up. Transpl Infect Dis. 2021; 23(4):e13663.
Rao R, Shetty AP, Nagesh CP. Orbital infarction syndrome secondary to rhino-orbital mucormycosis in a case of COVID-19: Clinico-radiological features. Indian J Ophthalmol. 2021; 69(6):1627-30.
Sharma S, Grover M, Bhargava S, Samdani S, Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol. 2021; 135(5):442-7.
Alekseyev K, Didenko L, Chaudhry B. Rhinocerebral Mucormycosis and COVID-19 Pneumonia. J Med Cases. 2021; 12 (3):85-9.
Bayram N, Ozsaygılı C, Sav H, et al. Susceptibility of severe COVID-19 patients to rhino-orbital mucormycosis fungal infection in different clinical manifestations. Jpn J Ophthalmol. 2021; 65(4):515-5.
Sen M, Lahane S, Lahane TP, Parekh R, Honavar SG. Mucor in a Viral Land: A Tale of Two Pathogens. Indian J Ophthalmol. 2021; 69(2):244-52.
Saldanha M, Reddy R, Vincent MJ. Title of the Article: Paranasal Mucormycosis in COVID-19 Patient. Indian J Otolaryngol Head Neck Surg. 2022; 74 (Suppl 2):3407-10.
Mekonnen ZK, Ashraf DC, Jankowski T, et al. Acute Invasive Rhino-Orbital Mucormycosis in a Patient with COVID-19-Associated Acute Respiratory Distress Syndrome. Ophthalmic Plast Reconstr Surg. 2021; 37(2):e40-e80.
Waizel-Haiat S, Guerrero-Paz JA, Sanchez-Hurtado L, Calleja-Alarcon S, Romero-Gutierrez L. A Case of Fatal Rhino-Orbital Mucormycosis Associated With New Onset Diabetic Ketoacidosis and COVID-19. Cureus. 2021; 13(2):e13163.
Garg D, Muthu V, Sehgal IS, et al. Coronavirus Disease (Covid-19) Associated Mucormycosis (CAM): Case Report and Systematic Review of Literature. Mycopathologia. 2021; 186(2):289-98.
Ravani SA, Agrawal GA, Leuva PA, Modi PH, Amin KD. Rise of the phoenix: Mucormycosis in COVID-19 times. Indian J Ophthalmol. 2021; 69(6):1563-8.
Veisi A, Bagheri A, Eshaghi M, Rikhtehgar MH, Rezaei Kanavi M, Farjad R. Rhino-orbital mucormycosis during steroid therapy in COVID-19 patients: A case report. Eur J Ophthalmol. 2022; 32(4):NP11-NP16.
Khatri A, Chang KM, Berlinrut I, Wallach F. Mucormycosis after Coronavirus disease 2019 infection in a heart transplant recipient – Case report and review of literature. J Mycol Med. 2021; 31(2):101125.
Maini A, Tomar G, Khanna D, Kini Y, Mehta H, Bhagyasree V. Sino-orbital mucormycosis in a COVID-19 patient: A case report. Int J Surg Case Rep. 2021;82:105957.
Khatri PM, Mittal M, Chand JS, Bhardwaj A, Khatri M. Rhino-orbital mucormycosis associated with COVID-19. Annals Rom Soc Cell Biol. 2021;19364-9.
Monte Junior ESD, Santos MELD, Ribeiro IB, et al. Rare and Fatal Gastrointestinal Mucormycosis (Zygomycosis) in a COVID-19 Patient: A Case Report. Clin Endosc. 2020; 53(6):746-9.
Nehara HR, Puri I, Singhal V, Ih S, Bishnoi BR, Sirohi P. Rhinocerebral mucormycosis in COVID-19 patient with diabetes a deadly trio: Case series from the north-western part of India. Indian J Med Microbiol. 2021; 39(3):380-3.
Revannavar SM, P S S, Samaga L, V K V. COVID-19 triggering mucormycosis in a susceptible patient: a new phenomenon in the developing world? BMJ Case Rep. 2021; 14(4):e241663.
Sarkar S, Gokhale T, Choudhury SS, Deb AK. COVID-19 and orbital mucormycosis [published correction appears in Indian J Ophthalmol. 2021 Jul; 69(7):1978. doi: 10.4103/0301-4738.318160]. Indian J Ophthalmol. 2021; 69(4):1002-4.
Sai Krishna D, Raj H, Kurup P, Juneja M. Maxillofacial Infections in Covid-19 Era-Actuality or the Unforeseen: 2 Case Reports. Indian J Otolaryngol Head Neck Surg. 2022; 74(Suppl 2):2959-62. doi:10.1007/s12070-021-02618-5
Pasero D, Sanna S, Liperi C, et al. A challenging complication following SARS-CoV-2 infection: a case of pulmonary mucormycosis. Infection. 2021; 49(5):1055-60.
Mekonnen ZK, Ashraf DC, Jankowski T, et al. Acute Invasive Rhino-Orbital Mucormycosis in a Patient with COVID-19-Associated Acute Respiratory Distress Syndrome. Ophthalmic Plast Reconstr Surg. 2021; 37(2):e40-e80.
Trovato L, Calvo M, Migliorisi G, Astuto M, Oliveri F, Oliveri S. Fatal VAP-related pulmonary aspergillosis by Aspergillus niger in a positive COVID-19 patient. Respir Med Case Rep. 2021; 32:101367.
Fouad YA, Abdelaziz TT, Askoura A, et al. Spike in Rhino-Orbital-Cerebral Mucormycosis Cases Presenting to a Tertiary Care Center During the COVID-19 Pandemic. Front Med (Lausanne). 2021; 8:645270.
Patel A, Agarwal R, Rudramurthy SM, et al. Multicenter Epidemiologic Study of Coronavirus Disease-Associated Mucormycosis, India. Emerg Infect Dis. 2021; 27(9):2349-59.
Meijer EFJ, Dofferhoff ASM, Hoiting O, Buil JB, Meis JF. Azole-Resistant COVID-19-Associated Pulmonary Aspergillosis in an Immunocompetent Host: A Case Report. J Fungi (Basel). 2020; 6(2):79.
Koehler P, Cornely OA, Böttiger BW, et al. COVID-19 associated pulmonary aspergillosis. Mycoses. 2020; 63(6):528-34.
Rutsaert L, Steinfort N, Van Hunsel T, et al. COVID-19-associated invasive pulmonary aspergillosis. Ann Intensive Care. 2020; 10(1):71.
Wu S, Yang S, Chen R, Chen H, Xu Y, Lin B. Dynamic Immune Response Profiles and Recovery of a COVID-19 Patient with Coinfection of Aspergillus fumigatus and Other Baseline Diseases: A Case Report. OMICS. 2020; 24(10):615-8.
Trovato L, Calvo M, Migliorisi G, Astuto M, Oliveri F, Oliveri S. Fatal VAP-related pulmonary aspergillosis by Aspergillus niger in a positive COVID-19 patient. Respir Med Case Rep. 2021; 32:101367.
Lamoth F, Glampedakis E, Boillat-Blanco N, Oddo M, Pagani JL. Incidence of invasive pulmonary aspergillosis among critically ill COVID-19 patients. Clin Microbiol Infect. 2020; 26(12):1706-8.
Antinori S, Rech R, Galimberti L, et al. Invasive pulmonary aspergillosis complicating SARS-CoV-2 pneumonia: A diagnostic challenge. Travel Med Infect Dis. 2020; 38:101752.
Mohamed A, Hassan T, Trzos-Grzybowska M, et al. Multi-triazole-resistant Aspergillus fumigatus and SARS-CoV-2 co-infection: A lethal combination. Med Mycol Case Rep. 2021; 31:11-4.
van Someren Gréve F, du Long R, Talwar R, Beurskens CJP, Voerman HJ, van Dijk K. Proven Fatal Invasive Aspergillosis in a Patient with COVID-19 and Staphylococcus aureus Pneumonia. J Fungi (Basel). 2021; 7(3):230.
Ghelfenstein-Ferreira T, Saade A, Alanio A, et al. Recovery of a triazole-resistant Aspergillus fumigatus in respiratory specimen of COVID-19 patient in ICU – A case report. Med Mycol Case Rep. 2021; 31:15-8.
Patti RK, Dalsania NR, Somal N, et al. Subacute Aspergillosis “Fungal Balls” Complicating COVID-19. J Investig Med High Impact Case Rep. 2020; 8:2324709620966475.
Alanio A, Dellière S, Fodil S, Bretagne S, Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med. 2020; 8(6):e48-e49.
Alobaid K, Yousuf B, Al-Qattan E, Muqeem Z, Al-Subaie N. Pulmonary aspergillosis in two COVID-19 patients from Kuwait. Access Microbiol. 2021; 3(3):000201.
Bartoletti M, Pascale R, Cricca M, et al. Epidemiology of Invasive Pulmonary Aspergillosis Among Intubated Patients With COVID-19: A Prospective Study. Clin Infect Dis. 2021; 73(11):e3606-e3614.
Mitaka H, Perlman DC, Javaid W, Salomon N. Pulmonary Aspergillosis in Critically Ill Patients with COVID-19: A Case Series. Open Forum Infect Dis. 2020;7:S613.
Santana MF, Pivoto G, Alexandre MAA, et al. Confirmed Invasive Pulmonary Aspergillosis and COVID-19: the value of postmortem findings to support antemortem management. Rev Soc Bras Med Trop. 2020; 53:e20200401.
Shadrach BJ, Goel R, Deokar K, Jain A. Invasive pulmonary aspergillosis in a COVID-19 recovered patient: unravelling an infective sequalae of the SARS-CoV-2 virus. Monaldi Arch Chest Dis. 2021; 91(2):10.4081/monaldi.2021.1664.
Sasoni N, Rodriguez Müller M, Posse G, González J, Leonardelli F, Garcia-Effron G. SARS-CoV-2 and Aspergillus section Fumigati coinfection in an immunocompetent patient treated with corticosteroids. Rev Iberoam Micol. 2021; 38(1):16-8.
Nasri E, Shoaei P, Vakili B, et al. Fatal Invasive Pulmonary Aspergillosis in COVID-19 Patient with Acute Myeloid Leukemia in Iran. Mycopathologia. 2020; 185(6):1077-84.
Borman AM, Palmer MD, Fraser M, et al. COVID-19-Associated Invasive Aspergillosis: Data from the UK National Mycology Reference Laboratory. J Clin Microbiol. 2020; 59(1):e02136-20. Published 2020 Dec 17.
Valente-Acosta B, Moreno-Sanchez F, Espinosa-Aguilar L, et al. Clinical characteristics of critically ill patients with COVID-19 and invasive pulmonary aspergillosis: a case series from Mexico City. Open Forum Infect Dis. 2020;7:S247-S248.
Vélez Pintado M, Camiro-Zúñiga A, Aguilar Soto M, et al. COVID-19-associated invasive pulmonary aspergillosis in a tertiary care center in Mexico City. Med Mycol. 2021; 59(8):828-33.
Abolghasemi S, Hakamifard A, Sharifynia S, Pourabdollah Toutkaboni M, Azhdari Tehrani H. Fatal invasive pulmonary aspergillosis in an immunocompetent patient with COVID-19 due to Aspergillusterreus: A case study. Clin Case Rep. 2021; 9(4):2414-8.
Trujillo H, Fernández‐Ruiz M, Gutiérrez E, et al. Invasive pulmonary aspergillosis associated Trujillo H, Fernández-Ruiz M, Gutiérrez E, et al. Invasive pulmonary aspergillosis associated with COVID-19 in a kidney transplant recipient. Transpl Infect Dis. 2021; 23(2):e13501.
Velez-Pintado M, Aguilar-Soto M, Camiro A, et al. Invasive aspergillosis in COVID-19 patients in an intensive care unit in Mexico City. Open Forum Infect Dis. 2020;7: S261-S261.
Benedetti MF, Alava KH, Sagardia J, et al. COVID-19 associated pulmonary aspergillosis in ICU patients: Report of five cases from Argentina. Med Mycol Case Rep. 2021; 31:24-8.
Helleberg M, Steensen M, Arendrup MC. Invasive aspergillosis in patients with severe COVID-19 pneumonia. Clin Microbiol Infect. 2021; 27(1):147-8.
Lahmer T, Kriescher S, Herner A, et al. Invasive pulmonary aspergillosis in critically ill patients with severe COVID-19 pneumonia: Results from the prospective AspCOVID-19 study. PLoS One. 2021; 16(3):e0238825.
Haglund A, Christensen S, Kristensen L, Gertsen JB, Buus L, Lausch KR. Invasive pulmonary aspergillosis and hyperthermia in an immunocompetent patient with COVID-19. Med Mycol Case Rep. 2021; 31:29-31.
Prattes J, Valentin T, Hoenigl M, Talakic E, Reisinger AC, Eller P. Invasive pulmonary aspergillosis complicating COVID-19 in the ICU – A case report. Med Mycol Case Rep. 2021; 31:2-5.
Nasrullah A, Javed A, Malik K. Coronavirus Disease-Associated Pulmonary Aspergillosis: A Devastating Complication of COVID-19. Cureus. 2021; 13(1):e13004.
Fernandez NB, Caceres DH, Beer KD, et al. Ventilator-associated pneumonia involving Aspergillus flavus in a patient with coronavirus disease 2019 (COVID-19) from Argentina. Med Mycol Case Rep. 2021; 31:19-23.
van Arkel ALE, Rijpstra TA, Belderbos HNA, van Wijngaarden P, Verweij PE, Bentvelsen RG. COVID-19-associated Pulmonary Aspergillosis. Am J Respir Crit Care Med. 2020; 202(1):132-5.
Imoto W, Himura H, Matsuo K, et al. COVID-19-associated pulmonary aspergillosis in a Japanese man: A case report. J Infect Chemother. 2021; 27(6):911-4.
Mitaka H, Perlman DC, Javaid W, Salomon N. Putative invasive pulmonary aspergillosis in critically ill patients with COVID-19: An observational study from New York City. Mycoses. 2020; 63(12):1368-72.
Abdalla S, Almaslamani MA, Hashim SM, Ibrahim AS, Omrani AS. Fatal Coronavirus Disease 2019-associated Pulmonary Aspergillosis; A Report of Two Cases and Review of the Literature. IDCases. 2020; 22:e00935.
Kakamad FH, Mahmood SO, Rahim HM, et al. Post covid-19 invasive pulmonary Aspergillosis: A case report. Int J Surg Case Rep. 2021; 82:105865.
Hakamifard A, Hashemi M, Fakhim H, Aboutalebian S, Hajiahmadi S, Mohammadi R. Fatal disseminated aspergillosis in an immunocompetent patient with COVID-19 due to Aspergillus ochraceus. J Mycol Med. 2021; 31(2):101124.
Dellière S, Dudoignon E, Fodil S, et al. Risk factors associated with COVID-19-associated pulmonary aspergillosis in ICU patients: a French multicentric retrospective cohort. Clin Microbiol Infect. Published online December 13, 2020.
Lahmer T, Rasch S, Spinner C, Geisler F, Schmid RM, Huber W. Invasive pulmonary aspergillosis in severe coronavirus disease 2019 pneumonia. Clin Microbiol Infect. 2020; 26(10):1428-9.
Spadea M, Carraro F, Saglio F, et al. Successfully treated severe COVID-19 and invasive aspergillosis in early hematopoietic cell transplantation setting. Transpl Infect Dis. 2021; 23(2):e13470.
Segrelles-Calvo G, Araújo GRS, Llopis-Pastor E, et al. Prevalence of opportunistic invasive aspergillosis in COVID-19 patients with severe pneumonia. Mycoses. 2021; 64(2):144-51.
Schein F, Munoz-Pons H, Mahinc C, Grange R, Cathébras P, Flori P. Fatal aspergillosis complicating severe SARS-CoV-2 infection: A case report. J Mycol Med. 2020; 30(4):101039.
Salehi M, Khajavirad N, Seifi A, et al. Proven Aspergillus flavus pulmonary aspergillosis in a COVID-19 patient: A case report and review of the literature. Mycoses. 2021; 64(8):809-16.
Wu S, Yang S, Chen R, Chen H, Xu Y, Lin B. Dynamic Immune Response Profiles and Recovery of a COVID-19 Patient with Coinfection of Aspergillus fumigatus and Other Baseline Diseases: A Case Report. OMICS. 2020; 24(10):615-8.
Blaize M, Mayaux J, Nabet C, et al. Fatal Invasive Aspergillosis and Coronavirus Disease in an Immunocompetent Patient. Emerg Infect Dis. 2020; 26(7):1636-7.
Versyck M, Zarrougui W, Lambiotte F, Elbeki N, Saint-Leger P. Invasive pulmonary aspergillosis in COVID-19 critically ill patients: Results of a French monocentric cohort. J Mycol Med. 2021; 31(2):101122.
Witting C, Quaggin-Smith J, Mylvaganam R, Peigh G, Angarone M, Flaherty JD. Invasive pulmonary aspergillosis after treatment with tocilizumab in a patient with COVID-19 ARDS: a case report. Diagn Microbiol Infect Dis. 2021; 99(4):115272.
Martín CS, Martíne EM, Pellicer RG, Ibanez RA, Gonzalez EM, Pitarch JVL. Invasive pulmonary aspergillosis in patients with acute respiratory syndrome by COVID-19. Rev Esp Anestesiol Reanim. 2021. doi:10.1016/j.redar.2021.02.012
Sharma A, Hofmeyr A, Bansal A, et al. COVID-19 associated pulmonary aspergillosis (CAPA): An Australian case report. Med Mycol Case Rep. 2021; 31:6-10.
Meijer EFJ, Dofferhoff ASM, Hoiting O, Meis JF. COVID-19-associated pulmonary aspergillosis: a prospective single-center dual case series. Mycoses. 2021; 64(4):457-64.
Wang J, Yang Q, Zhang P, Sheng J, Zhou J, Qu T. Clinical characteristics of invasive pulmonary aspergillosis in patients with COVID-19 in Zhejiang, China: a retrospective case series. Crit Care. 2020; 24(1):299.
Permpalung N, Chiang TP, Massie AB, et al. Coronavirus Disease 2019-Associated Pulmonary Aspergillosis in Mechanically Ventilated Patients. Clin Infect Dis. 2022; 74(1):83-91.
Er B, Er AG, Metan G, et al. Is COVID-19 a risk factor for invasive pulmonary aspergillosis in critically ill patients? Tuberk Toraks. 2021; 69(1):118-20.
Al-Hatmi AMS, Mohsin J, Al-Huraizi A, Khamis F. COVID-19 associated invasive candidiasis. J Infect. 2021; 82(2):e45-e46.
Baskaran V, Lawrence H, Lansbury LE, et al. Co-infection in critically ill patients with COVID-19: an observational cohort study from England. J Med Microbiol. 2021; 70(4):001350.
Bhagali R, Prabhudesai NP, Prabhudesai MN. Post COVID-19 opportunistic candida retinitis: A case report. Indian J Ophthalmol. 2021; 69(4):987-9.
Bishburg E, Okoh A, Nagarakanti SR, Lindner M, Migliore C, Patel P. Fungemia in COVID-19 ICU patients, a single medical center experience. J Med Virol. 2021; 93(5):2810-4.
Denny S, Abdolrasouli A, Elamin T, et al. A retrospective multicenter analysis of candidaemia among COVID-19 patients during the first UK pandemic wave. J Infect. 2021; 82(6):276-316.
Fekkar A, Lampros A, Mayaux J, et al. Occurrence of Invasive Pulmonary Fungal Infections in Patients with Severe COVID-19 Admitted to the ICU. Am J Respir Crit Care Med. 2021; 203(3):307-17.
Garcia-Vidal C, Sanjuan G, Moreno-García E, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021; 27(1):83-8.
White PL, Dhillon R, Cordey A, et al. A National Strategy to Diagnose Coronavirus Disease 2019-Associated Invasive Fungal Disease in the Intensive Care Unit. Clin Infect Dis. 2021; 73(7):e1634-e1644.
Kayaaslan B, Eser F, Kaya Kalem A, et al. Characteristics of candidemia in COVID-19 patients; increased incidence, earlier occurrence and higher mortality rates compared to non-COVID-19 patients. Mycoses. 2021; 64(9):1083-91.
Li Z, Chen ZM, Chen LD, et al. Coinfection with SARS-CoV-2 and other respiratory pathogens in patients with COVID-19 in Guangzhou, China. J Med Virol. 2020; 92(11):2381-3.
Nori P, Cowman K, Chen V, et al. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect Control Hosp Epidemiol. 2021; 42(1):84-8.
Nucci M, Barreiros G, Guimarães LF, Deriquehem VAS, Castiñeiras AC, Nouér SA. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses. 2021; 64(2):152-6.
Posteraro B, Torelli R, Vella A, et al. Pan-Echinocandin-Resistant Candida glabrata Bloodstream Infection Complicating COVID-19: A Fatal Case Report. J Fungi (Basel). 2020; 6(3):163.
Prestel C, Anderson E, Forsberg K, et al. Candida auris Outbreak in a COVID-19 Specialty Care Unit – Florida, July-August 2020. MMWR Morb Mortal Wkly Rep. 2021; 70(2):56-57.
Sari AP, Darnindro N, Yohanes A, Mokoagow MI. Role of tocilizumab for concomitant systemic fungal infection in severe COVID-19 patient: Case report. Medicine (Baltimore). 2021; 100(12):e25173.
Bellanger AP, Navellou JC, Lepiller Q, et al. Mixed mold infection with Aspergillus fumigatus and Rhizopus microsporus in a severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) patient. Infect Dis Now. 2021; 51(7):633-5.
Johnson AK, Ghazarian Z, Cendrowski KD, Persichino JG. Pulmonary aspergillosis and mucormycosis in a patient with COVID-19. Med Mycol Case Rep. 2021; 32:64-7.
Bhatt PJ, Shiau S, Brunetti L, et al. Risk Factors and Outcomes of Hospitalized Patients With Severe Coronavirus Disease 2019 (COVID-19) and Secondary Bloodstream Infections: A Multicenter Case-Control Study. Clin Infect Dis. 2021; 72(12):e995-e1003.
Ismaiel WF, Abdelazim MH, Eldsoky I, et al. The impact of COVID-19 outbreak on the incidence of acute invasive fungal rhinosinusitis. Am J Otolaryngol. 2021; 42(6):103080.
Buil JB, van Zanten ARH, Bentvelsen RG, et al. Case series of four secondary mucormycosis infections in COVID-19 patients, the Netherlands, December 2020 to May 2021. Euro Surveill. 2021; 26(23):2100510.
El-Kholy NA, El-Fattah AMA, Khafagy YW. Invasive Fungal Sinusitis in Post COVID-19 Patients: A New Clinical Entity. Laryngoscope. 2021; 131(12):2652-8.
Moorthy A, Gaikwad R, Krishna S, et al. SARS-CoV-2, Uncontrolled Diabetes and Corticosteroids-An Unholy Trinity in Invasive Fungal Infections of the Maxillofacial Region? A Retrospective, Multi-centric Analysis. J Maxillofac Oral Surg. 2021; 20(3):418-25.
Macauley P, Epelbaum O. Epidemiology and Mycology of Candidaemia in non-oncological medical intensive care unit patients in a tertiary center in the United States: Overall analysis and comparison between non-COVID-19 and COVID-19 cases. Mycoses. 2021; 64(6):634-40.
Riad A, Gomaa E, Hockova B, Klugar M. Oral candidiasis of COVID-19 patients: Case report and review of evidence. J Cosmet Dermatol. 2021; 20(6):1580-4.
Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, et al. Oral mucosal lesions in a COVID-19 patient: New signs or secondary manifestations? Int J Infect Dis. 2020; 97:326-8.
Heller HM, Gonzalez RG, Edlow BL, Ard KL, Gogakos T. Case 40-2020: A 24-Year-Old Man with Headache and Covid-19. N Engl J Med. 2020; 383(26):2572-80.
Menon AA, Berg DD, Brea EJ, et al. A Case of COVID-19 and Pneumocystis jirovecii Coinfection. Am J Respir Crit Care Med. 2020; 202(1):136-8.
Bhat P, Noval M, Doub JB, Heil E. Concurrent COVID-19 and Pneumocystis jirovecii pneumonia in a severely immunocompromised 25-year-old patient. Int J Infect Dis. 2020; 99:119-21.
Coleman H, Snell LB, Simons R, Douthwaite ST, Lee MJ. Coronavirus disease 2019 and Pneumocystis jirovecii pneumonia: a diagnostic dilemma in HIV. AIDS. 2020; 34(8):1258-60.
De Francesco MA, Alberici F, Bossini N, et al. Pneumocystis jirevocii and SARS-CoV-2 Co-Infection: A Common Feature in Transplant Recipients?. Vaccines (Basel). 2020; 8(3):544.
Alanio A, Dellière S, Voicu S, Bretagne S, Mégarbane B. The presence of Pneumocystis jirovecii in critically ill patients with COVID-19. J Infect. 2021; 82(4):84-123.
Jeican II, Inișca P, Gheban D, et al. COVID-19 and Pneumocystis jirovecii Pulmonary Coinfection-The First Case Confirmed through Autopsy. Medicina (Kaunas). 2021; 57(4):302.
Mang S, Kaddu-Mulindwa D, Metz C, et al. Pneumocystis jirovecii Pneumonia and Severe Acute Respiratory Syndrome Coronavirus 2 Coinfection in a Patient With Newly Diagnosed HIV-1 Infection. Clin Infect Dis. 2021; 72(8):1487-9.
de Macedo PM, Freitas AD, Bártholo TP, et al. Acute Pulmonary Histoplasmosis Following COVID-19: Novel Laboratorial Methods Aiding Diagnosis. J Fungi (Basel). 2021; 7(5):346.
Messina FA, Marin E, Caceres DH, et al. Coronavirus Disease 2019 (COVID-19) in a Patient with Disseminated Histoplasmosis and HIV-A Case Report from Argentina and Literature Review. J Fungi (Basel). 2020; 6(4):275.
Bertolini M, Mutti MF, Barletta JA, et al. COVID-19 associated with AIDS-related disseminated histoplasmosis: a case report. Int J STD AIDS. 2020; 31(12):1222-4.
Basso RP, Poester VR, Benelli JL, et al. COVID-19-Associated Histoplasmosis in an AIDS Patient. Mycopathologia. 2021; 186(1):109-12.
Chang CC, Senining R, Kim J, Goyal R. An Acute Pulmonary Coccidioidomycosis Coinfection in a Patient Presenting With Multifocal Pneumonia with COVID-19. J Investig Med High Impact Case Rep. 2020; 8:2324709620972244.
Woldie IL, Brown IG, Nwadiaro NF, et al. Autoimmune Hemolytic Anemia in a 24-Year-Old Patient With COVID-19 Complicated by Secondary Cryptococcemia and Acute Necrotizing Encephalitis: A Case Report and Review of Literature. J Med Cases. 2020; 11(11):362-5.
Ventoulis I, Sarmourli T, Amoiridou P, et al. Bloodstream Infection by Saccharomyces cerevisiae in Two COVID-19 Patients after Receiving Supplementation of Saccharomyces in the ICU. J Fungi (Basel). 2020; 6(3):98.
Poignon C, Blaize M, Vezinet C, Lampros A, Monsel A, Fekkar A. Invasive pulmonary fusariosis in an immunocompetent critically ill patient with severe COVID-19. Clin Microbiol Infect. 2020; 26(11):1582-4.
Nobrega de Almeida J Jr, Moreno L, Francisco EC, et al. Trichosporon asahii superinfections in critically ill COVID-19 patients overexposed to antimicrobials and corticosteroids. Mycoses. 2021; 64(8):817-22.
CDC. Candida auris. Atlanta, GA: US Department of Health and Human Services, CDC; 2020. Accessed 05 August 2021. https://www.cdc.gov/fungal/candida-auris/index.html.
Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020; 26(12):1622-9.
Sharma S, Singh A, Banerjee T. Antibacterial agents used in COVID-19: A systematic review and meta-analysis. Environ Sustain (Singap). 2021; 4(3):503-13.
Mucormycosis – WHO | World Health Organization. Coronavirus-disease-(covid-19) of COVID-19 associated mucormycosis and the Government of India. Available at: https://www.who.int/india/home/emergencies/coronavirus-disease-(covid-19)/mucormycosis#:~:text=Following%20the%20surge%20of%20COVID,and%20outcomes%20of%20these%20patients. (Accessed September 20, 2021.).
Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India. Diabetes Metab Syndr. 2021; 15(4):102146.
Moser D, Biere K, Han B, et al. COVID-19 Impairs Immune Response to Candida albicans. Front Immunol. 2021; 12:640644.
Arastehfar A, Carvalho A, Nguyen MH, et al. COVID-19-Associated Candidiasis (CAC): An Underestimated Complication in the Absence of Immunological Predispositions? J Fungi (Basel). 2020; 6(4):211.
Choy CY, Wong CS. It’s not all about COVID-19: pneumocystis pneumonia in the era of a respiratory outbreak. J Int AIDS Soc. 2020; 23(6):e25533.
Kelly S, Waters L, Cevik M, et al. Pneumocystis pneumonia, a COVID-19 mimic, reminds us of the importance of HIV testing in COVID-19. Clin Med (Lond). 2020; 20(6):590-2.
de Almeida Júnior JN, Hennequin C. Invasive Trichosporon Infection: a Systematic Review on a Re-emerging Fungal Pathogen. Front Microbiol. 2016; 7:1629.
Nobrega de Almeida J Jr, Moreno L, Francisco EC, et al. Trichosporon asahii superinfections in critically ill COVID-19 patients overexposed to antimicrobials and corticosteroids. Mycoses. 2021; 64(8):817-22.
Tomic Paradzik M, Mihic J, Kopic J, Missoni EM. Invasive Trichosporonosis in a Critically Ill ICU Patient: Case Report. Clin Microbiol. 2015;4:2.
Chowdhury MA, Hossain N, Kashem MA, Shahid MA, Alam A. Immune response in COVID-19: A review. J Infect Public Health. 2020; 13(11):1619-29.
Submit a Manuscript:
Copyright © Author(s) 2024. JASPI- Journal of Antimicrobial Stewardship Practices and Infectious Diseases.

